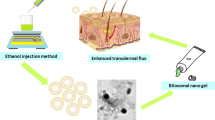
Abstract
Objective
Clinical utility of lornoxicam in oral therapy is primarily restricted by the low solubility and gastric adverse effects. This study evaluated the prospective of optimized proniosomal gel to improve the clinical efficacy of lornoxicam and compare with oral therapy.
Methods
Proniosomes were formulated by coacervation phase separation technique using span 60, lecithin and cholesterol. A four-factor three-level Box-Behnken design was used to evaluate the effect of amount of four independent variables; span 60 (X1), cholesterol (X2), lecithin (X3) and lornoxicam (X4) on response variables; vesicle size (Y1), entrapment efficiency (Y2) and transdermal flux (Y3). The selected proniosomal gel (F19) was characterized, and evaluated for the transdermal efficacy by ex vivo and in vivo experiments.
Results
Optimization study signifies that amount of formulation components (span 60, cholesterol, lecithin and lornoxicam) influence the vesicle size, entrapment efficiency and/or transdermal flux. Optimized formulation F19 exhibited nano size with high entrapment efficiency, adequate zeta potential, greater transdermal flux and better stability (at refrigerated conditions). The entrapment of lornoxicam in the bilayers of proniosome vesicles was confirmed by differential scanning calorimeter. Release profile of F19 was distinct (p < 0.001) from gel prepared using hydroxypropyl methylcellulose (control) and displayed steady lornoxicam release by Fickian diffusion. Transdermal administration of F19 significantly inhibited the carrageenan induced hind-paw edema in rats as compared to oral lornoxicam group.
Conclusions
The data observed in this study demonstrated that the developed proniosomal gel (F19) improved the clinical efficacy of lornoxicam as compared to oral therapy.

Proniosomal gel for transdermal delivery of lornoxicam: optimization using factorial design and in vivo evaluation in rats.








Similar content being viewed by others
References
Christian H, Jan GJ. Lornoxicam: pharmacology and usefulness to treat acute postoperative and musculoskeletal pain a narrative review. Expert Opin Pharmacother. 2013;14(12):1679–94.
Homdrum EM, Likar R, Nell G. Xefo® Rapid: a novel effective tool for pain treatment. Eur Surg. 2006;38:342–52.
Shahzad Y, Khan Q, Hussain T, Shah SNH. Influence of cellulose derivative and ethylene glycol on optimization of lornoxicam transdermal formulation. Int J Biol Macromol. 2013;61:26–32.
Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41(2):199–204.
Marwah H, Garg T, Rath G, Goyal AK. Development of transferosomal gel for trans-dermal delivery of insulin using iodine complex. Drug Deliv. 2016;23(5):1636–44.
Anroop B, Ghosh B, Parcha V, Khanam J. Transdermal delivery of atenolol: effect of prodrugs and iontophoresis. Curr Drug Deliv. 2009;6(3):280–90.
Dasgupta S, Ghosh SK, Ray S, Kaurav SS, Mazumder B. In vitro & in vivo studies on lornoxicam loaded nanoemulsion gels for topical application. Curr Drug Deliv. 2014;11(1):132–8.
Deepak K, Preeti W, Pradeep V. Niosomal gel of lornoxicam for topical delivery: in vitro assessment and pharmacodynamic activity. AAPS PharmSciTech. 2013;14(3):1072–82.
Li K, Gao S, Tian B, Shi Y, Lv Q, Han J. Formulation optimization and in-vitro and in-vivo evaluation of lornoxicam ethosomal gels with penetration enhancers. Curr Drug Deliv. 2018;15(3):424–35.
Nair AB, Chakraborty B, Murthy SN. Effect of polyethylene glycols on the trans-ungual delivery of terbinafine. Curr Drug Deliv. 2010;7(5):407–14.
Rehman K, Zulfakar MH. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev Ind Pharm. 2014;40(4):433–40.
Kamboj S, Nair A. Solid lipid nanoparticles: an effective lipid based technology for poorly water soluble drugs. Int J Pharm Sci Rev Res. 2010;5(2):78–90.
Khatoon M, Shah KU, Din FU, Shah SU, Rehman AU, Dilawar N, et al. Proniosomes derived niosomes: recent advancements in drug delivery and targeting. Drug Deliv. 2017;24(2):56–69.
Thakkar M. Opportunities and challenges for niosomes as drug delivery systems. Curr Drug Deliv. 2016;13(8):1275–89.
Ahmad MZ, Mohammed AA, Mokhtar Ibrahim M. Technology overview and drug delivery application of proniosome. Pharm Dev Technol. 2017;22(3):302–11.
Rahimpour Y, Kouhsoltani M, Hamishehkar H. Proniosomes in transdermal drug delivery. Curr Pharm Des. 2015;21(20):2883–91.
Madan JR, Ghuge NP, Dua K. Formulation and evaluation of proniosomes containing lornoxicam. Drug Deliv Transl Res. 2016;6(5):511–8.
Attimarad M, Rapid RP. HPLC method for quantitative determination of Lornoxicam in tablets. J Basic Clinical Pharm. 2010;1(2):115–8.
Alsarra IA. Evaluation of proniosomes as an alternative strategy to optimize piroxicam transdermal delivery. J Microencapsul. 2009;26:272–8.
Nair AB, Kaushik A, Attimarad M, Al-Dhubiab BE. Enhanced oral bioavailability of calcium using bovine serum albumin microspheres. Drug Deliv. 2012;19(6):277–85.
Nair AB, Jacob S, Al-Dhubiab BE, Alhumam RN. Influence of skin permeation enhancers on the transdermal delivery of palonosetron: an in vitro evaluation. J Appl Biomed. 2018;16(3):192–7.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Nair A, Vyas H, Shah J, Kumar A. Effect of permeation enhancers on the iontophoretic transport of metoprolol tartrate and the drug retention in skin. Drug Deliv. 2011;18(1):19–25.
Anroop B, Ghosh B, Parcha V, Kumar A, Khanam J. Synthesis and comparative skin permeability of atenolol and propranolol esters. J Drug Del Sci Tech. 2005;15(2):187–90.
Ammar HO, Ghorabb M, El-Nahhasc SA, Higazya IM. Proniosomes as a carrier system for transdermal delivery of tenoxicam. Int J Pharm. 2011;405:142–52.
Ustündağ Okur N, Apaydın S, Karabay Yavaşoğlu NÜ, Yavaşoğlu A, Karasulu HY. Evaluation of skin permeation and anti-inflammatory and analgesic effects of new naproxen microemulsion formulations. Int J Pharm. 2011;416(1):136–44.
Nair A, Morsy MA, Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 2018;79(8):373–82.
Azeem A, Jain N, Iqbal Z, Ahmad FJ, Aqil M, Talegaonkar S. Feasibility of proniosomes-based transdermal delivery of frusemide: formulation optimization and pharmacotechnical evaluation. Pharm Dev Technol. 2008;13(2):155–63.
Alsarra IA, Bosela AA, Ahmed SM, Mahrous GM. Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur J Pharm Biopharm. 2005;59:485–90.
Gannu R, Palem CR, Yamsani SK, Yamsani VV, Yamsani MR. Enhanced bioavailability of buspirone from reservoir-based transdermal therapeutic system, optimization of formulation employing box Behnken statistical design. AAPS PharmSciTech. 2010;11:976–85.
Chopra S, Patil GV, Motwani SK. Release modulating hydrophilic matrix systems of losartan potassium: optimization of formulation using statistical experimental design. Eur J Pharm Biopharm. 2007;66:73–82.
Hao Y, Zhao F, Li N, Yang Y, Li K. Studies on a high encapsulation of colchicine by a niosome system. Int J Pharm. 2002;244:73–80.
Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO, Oh DH, et al. Formulation and in-vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377:1–8.
El-Laithy HM, Shoukry O, Mahran LG. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J Pharm Biopharm. 2011;77:43–55.
EL-Samaligy MS, Afifi NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308:140–8.
Aboelwafa AA, Doaa AE, Aliaa NE. Comparative study on the effects of some polyoxyethylene alkyl ether and sorbitan fatty acid ester surfactants on the performance of transdermal carvedilol proniosomal gel using experimental design. AAPS PharmSciTech. 2010;11:1591–602.
Fang JY, Yu SY, Wu PC, Huang YB, Tsai YH. In-vitro skin permeation of estradiol from various proniosomes formulation. Int J Pharm. 2001;215:91–9.
Jacob S, Anroop B, Al-Dhubiab BE. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J Liposome Res. 2017;27(4):283–92.
El-Ridy MS, Yehia SA, Mohsen AM, El-Awdan SA, Darwish AB. Formulation of Niosomal gel for enhanced transdermal lornoxicam delivery: in-vitro and in-vivo evaluation. Curr Drug Deliv. 2018;15:122–33.
Acknowledgments
The authors are highly thankful to Arihant School of Pharmacy & BRI, Gandhinagar and Institute of Pharmacy, Nirma University, Ahmedabad for providing laboratory facilities. No financial support received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 540 kb)
Rights and permissions
About this article
Cite this article
Shah, H., Nair, A.B., Shah, J. et al. Proniosomal gel for transdermal delivery of lornoxicam: optimization using factorial design and in vivo evaluation in rats. DARU J Pharm Sci 27, 59–70 (2019). https://doi.org/10.1007/s40199-019-00242-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00242-x