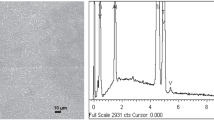
Abstract
Corrosion resistance is one of the essential factors in metal selection and can be enhanced greatly by coatings. The corrosion resistance of the electroless nickel–phosphorus coatings on low-carbon steels has been studied in this research.The scanning electron microscopy (SEM) and X-ray diffraction analysis (XRD) have been used to characterize the morphologies and compositions of the coatings.The corrosion behaviour of coated carbon steel has been evaluated bypotentiodynamic polarization and electrochemical impedance spectroscopy methods. Results show that the highest corrosion resistance of the coated substrate by the Ni‒P nano-structured coatingis achieved after heat treatment at 300°C.








Similar content being viewed by others
REFERENCES
Bozzini, B., Lenardi, C., Serra, M., and Fanigliulo, A., Electrochemical and X-ray photoelectron spectroscopy investigation into anodic behaviour of electroless Ni–9.5 wt % P in acidic chloride environment, Br. Corros. J., 2002, vol. 37, no. 3, p. 173.
Huang, C.-Y., Mo, W.-W., and Roan, M.-L., Studies on the influence of double-layer electroless metal deposition on the electromagnetic interference shielding effectiveness of carbon fiber/ABS composites, Surf. Coat. Technol., 2004, vol. 184, nos. 2–3, p. 163.
Rudnik, E., Kokoszka, K., and Łapsa, J., Comparative studies on the electroless deposition of Ni–P, Co–P and their composites with SiC particles, Surf. Coat. Technol., 2008, vol. 202, no. 12, p. 2584.
Khorasani, S.A.H. and Sanjabi, S., High corrosion resistance Ni-reduced graphene oxide nanocomposite coating, Corros. Rev., 2016, vol. 34, nos. 5–6, p. 305.
Farag, A.A., Kabel, K.I., Elnaggar, E.M., and Al-Gamal, A.G., Influence of polyaniline/multiwalled carbon nanotube composites on alkyd coatings against the corrosion of carbon steel alloy, Corros. Rev., 2017, vol. 35, no. 2, p. 85.
Voronov, V., Gubin, S., Cheglakov, A., Kornilov, D.Y., Karaseva, A., Krasnova, E., and Tkachev, S., Nanoparticles of complex oxides Li1 + x(NiyMnzCo1 ‒ y – z)1 – xO2 – δ (0≤ x ≤ 0.2, 0.2≤ y ≤ 0.6, 0.2≤ z ≤ 0.4) obtained by thermal destruction of metal-containing compounds in oil, Russ. J. Electrochem., 2017, vol. 53, no. 7, p. 769.
Chulkin, P., Ragoisha, G., and Streltsov, E., Platinum electrochemical corrosion and protection in concentrated alkali metal chloride solutions investigated by potentiodynamic nanogravimetry, Russ. J. Electrochem., 2017, vol. 53, no. 1, p. 1.
Balaji, S., Kumar, M.A., Manichandran, T., and Mutharasu, D., Electrodeposited three dimensional tin nano wire anode for thin film Li-ion micro batteries, Russ. J. Electrochem., 2016, vol. 52, no. 3, p. 226.
Ashassi-Sorkhabi, H. and Rafizadeh, S.H., Effect of coating time and heat treatment on structures and corrosion characteristics of electroless Ni–P alloy deposits, Surf. Coat. Technol., 2004, vol. 176, no. 3, p. 318.
Crobu, M., Scorciapino, A., Elsener, B., and Rossi, A., The corrosion resistance of electroless deposited nano-crystalline Ni–P alloys, Electrochim. Acta, 2008, vol. 53, no. 8, p. 3364.
Song, Y., Shan, D., and Han, E., High corrosion resistance of electroless composite plating coatings on AZ91D magnesium alloys, Electrochim. Acta, 2008, vol. 53, no. 5, p. 2135.
Alirezaei, S., Monirvaghefi, S., Salehi, M., and Saatchi, A., Wear behavior of Ni–P and Ni–P–Al2O3 electroless coatings, Wear, 2007, vol. 262, nos. 7–8, p. 978.
Wu, Y., Liu, H., Shen, B., Liu, L., and Hu, W., The friction and wear of electroless Ni–P matrix with PTFE and/or SiC particles composite, Tribol. Int., 2006, vol. 39, no. 6, p. 553.
Ebrahimian-Hosseinabadi, M., Azari-Dorcheh, K., and Vaghefi, S.M., Wear behavior of electroless Ni–P–B4C composite coatings, Wear, 2006, vol. 260, nos. 1–2, p. 123.
Novakovic, J., Vassiliou, P., Samara, K., and Argyropoulos, T., Electroless NiP–TiO2 composite coatings: their production and properties, Surf. Coat. Technol., 2006, vol. 201, no. 3-4, p. 895.
Straffelini, G., Colombo, D., and Molinari, A., Surface durability of electroless Ni–P composite deposits, Wear, 1999, vol. 236, no. 1-2, p. 179.
Chen, W., Gao, W., and He, Y., A novel electroless plating of Ni–P–TiO2 nano-composite coatings, Surf. Coat. Technol., 2010, vol. 204, no. 15, p. 2493.
Tamilselvi, M., Kamaraj, P., Arthanareeswari, M., Devikala, S., and Selvi, J.A., Development of nano SiO2 incorporated nano zinc phosphate coatings on mild steel, Appl. Surf. Sci., 2015, vol. 332, p. 12.
Gergely, A., Pászti, Z., Hakkel, O., Drotár, E., Mihály, J., and Kálmán, E., Corrosion protection of cold-rolled steel with alkyd paint coatings composited with submicron-structure types polypyrrole-modified nano-size alumina and carbon nanotubes, Mater. Sci. Eng. B, 2012, vol. 177, no. 18, p. 1571.
Sheng, M., Wang, Y., Zhong, Q., Wu, H., Zhou, Q., and Lin, H., The effects of nano-SiO2 additive on the zinc phosphating of carbon steel, Surf. Coat. Technol., 2011, vol. 205, no. 11, p. 3455.
Yang, Y., Chen, W., Zhou, C., Xu, H., and Gao, W., Fabrication and characterization of electroless Ni–P–ZrO2 nano-composite coatings, Appl. Nanosci., 2011, vol. 1, no. 1, p. 19.
Aal, A.A., El-Sheikh, S., and Ahmed, Y., Electrodeposited composite coating of Ni–W–P with nano-sized rod-and spherical-shaped SiC particles, Mater. Res. Bull., 2009, vol. 44, no. 1, p. 151.
Lu, J.H., Sun, W.C., Zhu, M., Tan, M.F., and Zhou, Q., Effects of content of Al2O3 particles and heat treatment on corrosion resistance of Ni–P–Al2O3 composite coatings, Adv. Mater. Res., 2010, vols. 105–106, no. 1, p. 441.
Tachev, D., Georgieva, J., and Armyanov, S., Magnetothermal study of nanocrystalline particle formation in amorphous electroless Ni–P and Ni–Me–P alloys, Electrochim. Acta, 2001, vol. 47, nos. 1–2, p. 359.
Yan, M., Ying, H., and Ma, T., Improved microhardness and wear resistance of the as-deposited electroless Ni–P coating, Surf. Coat. Technol., 2008, vol. 202, no. 24, p. 5909.
Abdoli, M. and Rouhaghdam, A.S., Preparation and characterization of Ni–P/nanodiamond coatings: effects of surfactants, Diamond Relat. Mater., 2013, vol. 31, p. 30.
Mallory, G.O. and Hajdu, J.B., Electroless Plating: Fundamentals and Applications, William Andrew, 1990.
International, A., Committee, A.I.H., and Committee, A.I.A.P.D., Metals Handbook: Properties and Selection, ASM Int., 1990.
Williamson, G. and Hall, W., X-ray line broadening from filed aluminium and wolfram, Acta Metall., 1953, vol. 1, no. 1, p. 22.
Qazi, S.J.S., Rennie, A.R., Cockcroft, J.K., and Vickers, M., Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles, J. Colloid Interface Sci., 2009, vol. 338, no. 1, p. 105.
Bushroa, A., Rahbari, R., Masjuki, H., and Muhamad, M., Approximation of crystallite size and microstrain via XRD line broadening analysis in TiSiN thin films, Vacuum, 2012, vol. 86, no. 8, p. 1107.
Sarma, H. and Sarma, K., X-ray peak broadening analysis of ZnO nanoparticles derived by precipitation method, Int. J. Sci. Res. Publ., 2014, vol. 4, no. 3, p. 1.
Habazaki, H., Ding, S.-Q., Kawashima, A., Asami, K., Hashimoto, K., Inoue, A., and Masumoto, T., The anodic behavior of amorphous Ni–19P alloys in different amorphous states, Corros. Sci., 1989, vol. 29, nos. 11–12, p. 1319.
Carbajal, J.L. and White, R.E., Electrochemical production and corrosion testing of amorphous Ni–P, J. Electrochem. Soc., 1988, vol. 135, no. 12, p. 2952.
Balaraju, J., Narayanan, T.S., and Seshadri, S., Evaluation of the corrosion resistance of electroless Ni–P and Ni–P composite coatings by electrochemical impedance spectroscopy, J. Solid State Electrochem., 2001, vol. 5, no. 5, p. 334.
Balaraju, J., Selvi, V.E., Grips, V.W., and Rajam, K., Electrochemical studies on electroless ternary and quaternary Ni–P based alloys, Electrochim. Acta, 2006, vol. 52, no. 3, p. 1064.
Stern, M. and Geary, A.L., Electrochemical polarization I. A theoretical analysis of the shape of polarization curves, J. Electrochem. Soc., 1957, vol. 104, no. 1, p. 56.
Rybalka, K., Beketaeva, L., and Davydov, A., Cathodic component of corrosion process: polarization curve with two tafel portions, Russ. J. Electrochem., 2018, vol. 54, no. 5, p. 456.
Rybalka, K., Beketaeva, L., and Davydov, A., Corrosion behavior of aluminum in 1 M HCl solution, Russ. J. Electrochem., 2016, vol. 52, no. 5, p. 463.
Rybalka, K., Beketaeva, L., and Davydov, A., Determination of corrosion current density by the rate of cathodic depolarizer consumption, Russ. J. Electrochem., 2016, vol. 52, no. 3, p. 268.
Veloz, M. and González, I., Electrochemical study of carbon steel corrosion in buffered acetic acid solutions with chlorides and H2S, Electrochim. Acta, 2002, vol. 48, no. 2, p. 135.
Mansfeld, F., Electrochemical impedance spectroscopy (EIS) as a new tool for investigating methods of corrosion protection, Electrochim. Acta, 1990, vol. 35, no. 10, p. 1533.
ACKNOWLEDGMENTS
This research has been performed using the facilities by Shiraz Branch, Islamic Azad University, Shiraz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue and I, as the corresponding author has no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Rights and permissions
About this article
Cite this article
Esmaeil Jafari Improving the Corrosion Resistance of Carbon Steel by Ni–P Nano-Structured Coating. Russ J Electrochem 57, 663–670 (2021). https://doi.org/10.1134/S1023193520120083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520120083