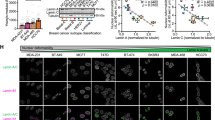
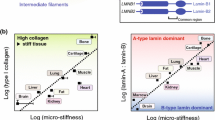
Abstract
One of the main factors associated with worse prognosis in oncology is metastasis, which is based on the ability of tumor cells to migrate from the primary source and to form secondary tumors. The search for new strategies to control migration of metastatic cells is one of the urgent issues in biomedicine. One of the strategies to stop spread of cancer cells could be regulation of the nuclear elasticity. Nucleus, as the biggest and stiffest cellular compartment, determines mechanical properties of the cell as a whole, and, hence, could prevent cell migration through the three-dimensional extracellular matrix. Nuclear rigidity is maintained by the nuclear lamina, two-dimensional network of intermediate filaments in the inner nuclear membrane (INM). Here we present the most significant factors defining nucleus rigidity, discuss the role of nuclear envelope composition in the cell migration, as well consider possible approaches to control lamina composition in order to change plasticity of the cell nucleus and ability of the tumor cells to metastasize.
Similar content being viewed by others
Abbreviations
- ER:
-
endoplasmic reticulum
- HGPS:
-
Hutchinson–Guildford Progeria
- LBR:
-
Lamin B receptor
- nt:
-
nucleotide
- ZMPSTE24:
-
zinc metallopeptidase STE24
References
Raab, M., Gentili, M., de Belly, H., Thiam, H.-R., Vargas, P., et al. (2016) ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death, Science, 352, 359-362, https://doi.org/10.1126/science.aad7611.
Lämmermann, T., Bader, B.L., Monkley, S. J., Worbs, T., Wedlich-Söldner, R., et al. (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing, Nature, 453, 51-55, https://doi.org/10.1038/nature06887.
Denais, C.M., Gilbert, R. M., Isermann, P., McGregor, A. L., te Lindert, M., et al. (2016) Nuclear envelope rupture and repair during cancer cell migration, Science, 352, 353-358, https://doi.org/10.1126/science.aad7297.
Caille, N., Thoumine, O., Tardy, Y., and Meister, J.-J. (2002) Contribution of the nucleus to the mechanical properties of endothelial cells, J. Biomech., 35, 177-187, https://doi.org/10.1016/S0021-9290(01)00201-9.
Guilak, F., Tedrow, J. R., and Burgkart, R. (2000) Viscoelastic properties of the cell nucleus, Biochem. Biophys. Res. Commun., 269, 781-786, https://doi.org/10.1006/bbrc.2000.2360.
Shankar, A., Syamala, S., and Kalidindi, S. (2010) Insufficient rest or sleep and its relation to cardiovascular disease, diabetes and obesity in a national, multiethnic sample, PLoS One, 5, e14189, https://doi.org/10.1371/journal.pone.0014189.
Wolf, K., te Lindert, M., Krause, M., Alexander, S., te Riet, J., et al. (2013) Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force, J. Cell Biol., 201, 1069-1084, https://doi.org/10.1083/jcb.201210152.
Finlan, L.E., and Bickmore, W.A. (2008) Porin new light onto chromatin and nuclear organization, Genome Biol., 9, 222, https://doi.org/10.1186/gb-2008-9-5-222.
Mewborn, S. K., Puckelwartz, M. J., Abuisneineh, F., Fahrenbach, J. P., Zhang, Y., et al. (2010) Altered chromosomal positioning, compaction, and gene expression with a lamin A/C gene mutation, PLoS One, 5, e14342, https://doi.org/10.1371/journal.pone.0014342.
Dechat, T., Adam, S. A., Taimen, P., Shimi, T., and Goldman, R. D. (2010) Nuclear lamins, Cold Spring Harb. Perspect. Biol., 2, 1-23, https://doi.org/10.1101/cshperspect.a000547.
Schirmer, E.C., Guan, T., and Gerace, L. (2001) Involvement of the lamin rod domain in heterotypic lamin interactions important for nuclear organization, J. Cell Biol., 152, 479-489, https://doi.org/10.1083/jcb.153.3.479.
Fuchs, E., Weber, K. (1994) Intermediate filaments: structure, dynamics, function and disease, Annu. Rev. Biochem., 63, 345-82, https://doi.org/10.1146/annurev.bi.63.070194.002021.
McKeon, F. D., Kirschner, M. W., and Caput, D. (1986) Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins, Nature, 319, 463-468, https://doi.org/10.1038/319463a0.
Rusiñol, A. E., Sinensky, M. S. (2006) Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors, J. Cell Sci., 119, 3265-3272, https://doi.org/10.1242/jcs.03156.
Adam, S.A., Butin-Israeli, V., Cleland, M. M., Shimi, T., and Goldman, R. D. (2013) Disruption of lamin B1 and lamin B2 processing and localization by farnesyltransferase inhibitors, Nucleus, 4, 142-150, https://doi.org/10.4161/nucl.24089.
Güttinger, S., Laurell, E., and Kutay, U. (2009) Orchestrating nuclear envelope disassembly and reassembly during mitosis, Nat. Rev. Mol. Cell Biol., 10, 178-191, https://doi.org/10.1038/nrm2641.
Aebi, U., Cohn, J., Buhle, L., Gerace, L. (1986) The nuclear lamina is a meshwork of intermediate-type filaments, Nature, 323, 560-564, https://doi.org/10.1038/323560a0.
Hennekes, H., Nigg, E.A. (1994) The role of isoprenylation in membrane attachment of nuclear lamins. A single point mutation prevents proteolytic cleavage of the lamin A precursor and confers membrane binding properties, J. Cell Sci., 107, 1019-29.
Stuurman, N., Heins, S., and Aebi, U. (1998) Nuclear lamins: their structure, assembly, and interactions, J. Struct. Biol., 122, 42-66, https://doi.org/10.1006/jsbi.1998.3987.
Shimi, T., Butin-Israeli, V., Adam, S. A., and Goldman, R. D. (2010) Nuclear lamins in cell regulation and disease, Cold Spring Harb. Symp. Quant. Biol., 75, 525-531, https://doi.org/10.1101/sqb.2010.75.045.
Turgay, Y., Eibauer, M., Goldman, A. E., Shimi, T., Khayat, M., et al. (2017) The molecular architecture of lamins in somatic cells, Nature, 543, 261-264, https://doi.org/10.1038/nature21382.
Shimi, T., Pfleghaar, K., Kojima, S. I., Pack, C. G., Solovei, I., et al. (2008) The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription, Genes Dev., 22, 3409-3421, https://doi.org/10.1101/gad.1735208.
Zhironkina, O. A., Kurchashova, S. Y., Pozharskaia, V. A., Cherepanynets, V. D., Strelkova, O. S., et al. (2016) Mechanisms of nuclear lamina growth in interphase, Histochem. Cell Biol., 145, 419-432, https://doi.org/10.1007/s00418-016-1419-6.
Cho, S., Vashisth, M., Abbas, A., Majkut, S., Vogel, K., Xia, Y., et al. (2019) Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest, Dev. Cell, 49, 920-935, https://doi.org/10.1016/j.devcel.2019.04.020.
Irianto, J., Pfeifer, C. R., Ivanovska, I. L., Swift, J., and Discher, D. E. (2016) Nuclear lamins in cancer, Cell. Mol. Bioeng., 9, 258-267, https://doi.org/10.1007/s12195-016-0437-8.
Swift, J., Ivanovska, I. L., Buxboim, A., Harada, T., Dingal, P. C. D. P., et al. (2013) Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation, Science, 341, 1240104, https://doi.org/10.1126/science.1240104.
Pajerowski, J. D., Dahl, K. N., Zhong, F. L., Sammak, P. J., and Discher, D. E. (2007) Physical plasticity of the nucleus in stem cell differentiation, Proc. Natl. Acad. Sci. USA, 104, 15619-24, https://doi.org/10.1073/pnas.0702576104.
Lammerding, J., Fong, L. G., Ji, J. Y., Reue, K., Stewart, C. L., et al. (2006) Lamins A and C but Not Lamin B1 regulate nuclear mechanics, J. Biol. Chem., 281, 25768-25780, https://doi.org/10.1074/jbc.M513511200.
Jung, H.J., Coffinier, C., Choe, Y., Beigneux, A. P., Davies, B. S. J., et al. (2012) Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA, Proc. Natl. Acad. Sci. USA, 109, E423-31, https://doi.org/10.1073/pnas.1111780109.
Constantinescu, D., Gray, H. L., Sammak, P. J., Schatten, G. P., and Csoka, A. B. (2006) Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation, Stem Cells, 24, 177-185, https://doi.org/10.1634/stemcells.2004-0159.
Pekovic, V., and Hutchison, C. J. (2008) Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches, J. Anat., 213, 5-25, https://doi.org/10.1111/j.1469-7580.2008.00928.x.
Mounkes, L.C., Kozlov, S., Hernandez, L., Sullivan, T., and Stewart, C. L. (2003) A progeroid syndrome in mice is caused by defects in A-type lamins, Nature, 423, 298-301, https://doi.org/10.1038/nature01631.
Prather, R. S., Kubiak, J., Maul, G. G., First, N. L., and Schatten, G. (1991) The expression of nuclear lamin A and C epitopes is regulated by the developmental stage of the cytoplasm in mouse oocytes or embryos, J. Exp. Zool., 257, 110-114, https://doi.org/10.1002/jez.1402570114.
Rober, R.A., Sauter, H., Weber, K., and Osborn, M. (1990) Cells of the cellular immune and hemopoietic system of the mouse lack lamins A/C: distinction versus other somatic cells, J. Cell Sci., 95, 587-598.
Schäpe, J., Prauße, S., Radmacher, M., and Stick, R. (2009) Influence of lamin A on the mechanical properties of amphibian oocyte nuclei measured by atomic force microscopy, Biophys. J., 96, 4319-4325, https://doi.org/10.1016/j.bpj.2009.02.048.
Harada, T., Swift, J., Irianto, J., Shin, J.-W., Spinler, K. R., et al. (2014) Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival, J. Cell Biol., 204, 669-682, https://doi.org/10.1083/jcb.201308029.
Rowat, A. C., Jaalouk, D. E., Zwerger, M., Ung, W. L., Eydelnant, I. A., et al. (2013) Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions, J. Biol. Chem., 288, 8610-8618, https://doi.org/10.1074/jbc.M112.441535.
Shah, P., Hobson, C. M., Cheng, S., Colville, M. J., Paszek, M. J., et al. (2021) Nuclear deformation causes DNA damage by increasing replication stress, Curr. Biol., 31, 753-765, https://doi.org/10.1016/j.cub.2020.11.037.
Olins, A. L., Herrmann, H., Lichter, P., Kratzmeier, M., Doenecke, D., and Olins, D. E. (2001) Nuclear Envelope and chromatin compositional differences comparing undifferentiated and retinoic acid- and phorbol ester-treated HL-60 cells, Exp. Cell Res., 268, 115-127, https://doi.org/10.1006/excr.2001.5269.
Pillay, J., den Braber, I., Vrisekoop, N., Kwast, L. M., de Boer, R. J., et al. (2010) In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days, Blood, 116, 625-627, https://doi.org/10.1182/blood-2010-01-259028.
Shin, J.W., Swift, J., Ivanovska, I., Spinler, K. R., Buxboim, A., and Discher, D. E. (2013) Mechanobiology of bone marrow stem cells: from myosin-II forces to compliance of matrix and nucleus in cell forms and fates, Differentiation, 86, 77-86, https://doi.org/10.1016/j.diff.2013.05.001.
Kittisopikul, M., Shimi, T., Tatli, M., Tran, J. R., Zheng, Y., et al. (2021) Computational analyses reveal spatial relationships between nuclear pore complexes and specific lamins, J. Cell Biol., 220, e202007082, https://doi.org/10.1083/JCB.202007082.
Moir, R. D., Spann, T. P., Herrmann, H., and Goldman, R. D. (2000) Disruption of nuclear lamin organization blocks the elongation phase of DNA replication, J. Cell Biol., 149, 1179-91, https://doi.org/10.1083/jcb.149.6.1179.
Vergnes, L., Péterfy, M., Bergo, M. O., Young, S. G., and Reue, K. (2004) Lamin B1 is required for mouse development and nuclear integrity, Proc. Natl. Acad. Sci. USA, 101, 10428-33, https://doi.org/10.1073/pnas.0401424101.
Coffinier, C., Jung, H. J., Nobumori, C., Chang, S., Tu, Y., et al. (2011) Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons, Mol. Biol. Cell, 22, 4461-4703, https://doi.org/10.1091/mbc.E11-06-0504.
Chen, N. Y., Yang, Y., Weston, T. A., Belling, J. N., Heizer, P., et al. (2019) An absence of lamin B1 in migrating neurons causes nuclear membrane ruptures and cell death, Proc. Natl. Acad. Sci. USA, 116, 25870-25879, https://doi.org/10.1073/pnas.1917225116.
Banigan, E. J., Stephens, A. D., Marko, J. F. (2017) Mechanics and buckling of biopolymeric shells and cell nuclei, Biophys. J., 113, 1654-63, https://doi.org/10.1016/j.bpj.2017.08.034.
Ou, H. D., Phan, S., Deerinck, T. J., Thor, A., Ellisman, M. H., and O’Shea, C. C. (2017) ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells, Science, 357, eaag0025, https://doi.org/10.1126/science.aag0025.
Bouck, D. C., and Bloom, K. (2007) Pericentric Chromatin is an elastic component of the mitotic spindle, Curr. Biol., 17, 741-748, https://doi.org/10.1016/j.cub.2007.03.033.
Stephens, A. D., Banigan, E. J., Adam, S. A., Goldman, R. D., and Marko, J. F. (2017) Chromatin and lamin a determine two different mechanical response regimes of the cell nucleus, Mol. Biol. Cell, 28, 1984-1996, https://doi.org/10.1091/mbc.E16-09-0653.
Booth-Gauthier, E. A., Alcoser, T. A., Yang, G., and Dahl, K. N. (2012) Force-induced changes in subnuclear movement and rheology, Biophys. J., 103, 2423-2431, https://doi.org/10.1016/j.bpj.2012.10.039.
Chalut, K. J., Höpfler, M., Lautenschläger, F., Boyde, L., Chan, C. J., et al. (2012) Chromatin decondensation and nuclear softening accompany Nanog downregulation in embryonic stem cells, Biophys. J., 103, 2060-2070, https://doi.org/10.1016/j.bpj.2012.10.015.
Spagnol, S. T., and Dahl, K. N. (2016) Spatially resolved quantification of chromatin condensation through differential local rheology in cell nuclei fluorescence lifetime imaging, PLoS One, 11, e0146244, https://doi.org/10.1371/journal.pone.0146244.
Gerace, L., and Tapia, O. (2018) Messages from the voices within: regulation of signaling by proteins of the nuclear lamina, Curr. Opin. Cell Biol., 52, 14-21, https://doi.org/10.1016/j.ceb.2017.12.009.
Koch, A. J., and Holaska, J. M. (2014) Emerin in health and disease, Semin. Cell Dev. Biol., 29, 95-106, https://doi.org/10.1016/j.semcdb.2013.12.008.
Bouzid, T., Kim, E., Riehl, B. D., Esfahani, A. M., Rosenbohm, J., et al. (2019) The LINC complex, mechanotransduction, and mesenchymal stem cell function and fate, J. Biol. Eng., 13, 1-12, https://doi.org/10.1186/s13036-019-0197-9.
Buxboim, A., Irianto, J., Swift, J., Athirasala, A., Shin, J.-W., et al. (2017) Coordinated increase of nuclear tension and lamin-A with matrix stiffness outcompetes lamin-B receptor that favors soft tissue phenotypes, Mol. Biol. Cell, 28, 3333-3348, https://doi.org/10.1091/mbc.E17-06-0393.
Wu, D., Flannery, A.R., Cai, H., Ko, E., and Cao, K. (2014) Nuclear localization signal deletion mutants of lamin A and progerin reveal insights into lamin A processing and emerin targeting, Nucleus, 5, 66-74, https://doi.org/10.4161/nucl.28068.
Eisch, V., Lu, X., Gabriel, D., and Djabali, K. (2016) Progerin impairs chromosome maintenance by depleting CENP-F from metaphase kinetochores in Hutchinson–Gilford progeria fibroblasts, Oncotarget, 7, 24700-24718, https://doi.org/10.18632/oncotarget.8267.
Chen, Z.-J., Wang, W.-P., Chen, Y.-C., Wang, J.-Y., Lin, W.-H., et al. (2014) Dysregulated interactions between lamin A and SUN1 induce abnormalities in the nuclear envelope and endoplasmic reticulum in progeric laminopathies, J. Cell Sci., 127, 1792-1804, https://doi.org/10.1242/jcs.139683.
Chang, W., Wang, Y., Luxton, G. W. G., Östlund, C., Worman, H. J., and Gundersen, G. G. (2019) Imbalanced nucleocytoskeletal connections create common polarity defects in progeria and physiological aging, Proc. Natl. Acad. Sci. USA, 116, 3578-3583, https://doi.org/10.1073/pnas.1809683116.
Subramanian, G., Chaudhury, P., Malu, K., Fowler, S., Manmode, R., et al. (2012) Lamin B receptor regulates the growth and maturation of myeloid progenitors via its sterol reductase domain: implications for cholesterol biosynthesis in regulating myelopoiesis, J. Immunol., 188, 85-102, https://doi.org/10.4049/jimmunol.1003804.
Solovei, I., Wang, A. S., Thanisch, K., Schmidt, C. S., Krebs, S., et al. (2013) LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation, Cell, 152, 584-598, https://doi.org/10.1016/j.cell.2013.01.009.
Zink, D., Fischer, A. H., Nickerson, J. A. (2004) Nuclear structure in cancer cells, Nat. Rev. Cancer, 4, 677-87, https://doi.org/10.1038/nrc1430.
Hudson, M.E., Pozdnyakova, I., Haines, K., Mor, G., and Snyder, M. (2007) Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays, Proc. Natl. Acad. Sci. USA, 104, 17494-9, https://doi.org/10.1073/pnas.0708572104.
Tilli, C. M. L. J., Ramaekers, F. C. S., Broers, J. L. V., Hutchison, C. J., and Neumann, H. A. M. (2003) Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma, Br. J. Dermatol., 148, 102-109, https://doi.org/10.1046/j.1365-2133.2003.05026.x.
Willis, N. D., Cox, T. R., Rahman-Casañs, S. F., Smits, K., Przyborski, S. A., et al. (2008) Lamin A/C is a risk biomarker in colorectal cancer, PLoS One, 3, e2988, https://doi.org/10.1371/journal.pone.0002988.
Venables, R. S., McLean, S., Luny, D., Moteleb, E., Morley, S., et al. (2001) Expression of individual lamins in basal cell carcinomas of the skin, Br. J. Cancer, 84, 512-519, https://doi.org/10.1054/bjoc.2000.1632.
Agrelo, R., Setien, F., Espada, J., Artiga, M. J., Rodriguez, M., et al. (2005) Inactivation of the lamin A/C gene by cpg island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-Cell lymphoma, J. Clin. Oncol., 23, 3940-3947, https://doi.org/10.1200/JCO.2005.11.650.
Alhudiri, I. M., Nolan, C. C., Ellis, I. O., Elzagheid, A., Rakha, E. A., et al. (2019) Expression of Lamin A/C in early-stage breast cancer and its prognostic value, Breast Cancer Res. Treat., 174, 661-8, https://doi.org/10.1007/s10549-018-05092-w.
Moss, S. F., Krivosheyev, V., de Souza, A., Chin, K., Gaetz, H. P., et al. (1999) Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms, Gut, 45, 723-729, https://doi.org/10.1136/gut.45.5.723.
Broers, J. L. V., Peeters, E. A. G., Kuijpers, H. J. H., Endert, J., Bouten, C. V. C., et al. (2004) Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies, Hum. Mol. Genet., 13, 2567-2580, https://doi.org/10.1093/hmg/ddh295.
Wong, K.-F., and Luk, J. M. (2012) in Liver Proteomics, Humana Press, Totowa, NJ, pp. 295-310.
Belt, E. J. T., Fijneman, R. J. A., van den Berg, E. G., Bril, H., Delis-van Diemen, P. M., et al. (2011) Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence, Eur. J. Cancer, 47, 1837-1845, https://doi.org/10.1016/j.ejca.2011.04.025.
Friedl, P., Wolf, K., and Lammerding, J. (2011) Nuclear mechanics during cell migration, Curr. Opin. Cell Biol., 23, 55-64, https://doi.org/10.1016/j.ceb.2010.10.015.
Van Helvert, S., Storm, C., Friedl, P. (2018) Mechanoreciprocity in cell migration, Nat. Cell Biol., 20, 8-20, https://doi.org/10.1038/s41556-017-0012-0.
Kaspi, E., Frankel, D., Guinde, J., Perrin, S., Laroumagne, S., et al. (2017) Low lamin A expression in lung adenocarcinoma cells from pleural effusions is a pejorative factor associated with high number of metastatic sites and poor performance status, PLoS One, 12, e0183136, https://doi.org/10.1371/journal.pone.0183136.
Khan, Z. S., Santos, J. M., and Hussain, F. (2018) Aggressive prostate cancer cell nuclei have reduced stiffness, Biomicrofluidics, 12, 014102, https://doi.org/10.1063/1.5019728.
Wang, Y., Jiang, J., He, L., Gong, G., and Wu, X. (2019) Effect of lamin-A expression on migration and nuclear stability of ovarian cancer cells, Gynecol. Oncol., 152, 166-176, https://doi.org/10.1016/j.ygyno.2018.10.030.
Mitchell, M. J., Denais, C., Chan, M. F., Wang, Z., Lammerding, J., and King, M. R. (2015) Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress, Am. J. Physiol. Physiol., 309, C736-746, https://doi.org/10.1152/ajpcell.00050.2015.
Roncato, F., Regev, O., Feigelson, S. W., Yadav, S. K., Kaczmarczyk, L., et al. (2021) Reduced lamin A/C Does not facilitate cancer cell transendothelial migration but compromises lung metastasis, Cancers (Basel), 13, 2383, https://doi.org/10.3390/cancers13102383.
Jia, Y., Vong, J. S.-L., Asafova, A., Garvalov, B. K., Caputo, L., et al. (2019) Lamin B1 loss promotes lung cancer development and metastasis by epigenetic derepression of RET, J. Exp. Med., 216, 1377-1395, https://doi.org/10.1084/jem.20181394.
Guelen, L., Pagie, L., Brasset, E., Meuleman, W., Faza, M. B., et al. (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions, Nature, 453, 948-951, https://doi.org/10.1038/nature06947.
Shah, P. P., Donahue, G., Otte, G. L., Capell, B. C., Nelson, D. M., et al. (2013) Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape, Genes Dev., 27, 1787-99, https://doi.org/10.1101/gad.223834.113.
Nguyen, A. V., Nyberg, K. D., Scott, M. B., Welsh, A. M., Nguyen, A. H., et al. (2016) Stiffness of pancreatic cancer cells is associated with increased invasive potential, Integr. Biol., 8, 1232-1245, https://doi.org/10.1039/C6IB00135A.
Kong, L., Schäfer, G., Bu, H., Zhang, Y., Zhang, Y., and Klocker, H. (2012) Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway, Carcinogenesis, 33, 751-759, https://doi.org/10.1093/carcin/bgs022.
Zhang, X., and Lv, Y. (2017) Suspension state increases reattachment of breast cancer cells by up-regulating lamin A/C, Biochim. Biophys. Acta Mol. Cell Res., 1864, 2272-2282, https://doi.org/10.1016/j.bbamcr.2017.09.006.
Li, L., Du, Y., Kong, X., Li, Z., Jia, Z., et al. (2013) Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer, Clin. Cancer Res., 19, 4651-4561, https://doi.org/10.1158/1078-0432.CCR-12-3630.
Liu, L., Wang, J., Shi, L., Zhang, W., Du, X., et al. (2013) β-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression, Phytomedicine, 20, 512-20, https://doi.org/10.1016/j.phymed.2012.12.008.
Fracchia, A., Asraf, T., Salmon-Divon, M., and Gerlitz, G. (2020) Increased lamin B1 levels promote cell migration by altering perinuclear actin organization, Cells, 9, 2161, https://doi.org/10.3390/cells9102161.
Béroud, C., Collod‐Béroud, G., Boileau, C., Soussi, T., and Junien, C. (2000) UMD (Universal Mutation Database): a generic software to build and analyze locus-specific databases, Hum. Mutat., 15, 86-94, https://doi.org/10.1002/(SICI)1098-1004(200001)15:1<86::AID-HUMU16>3.0.CO;2-4.
Padiath, Q.S., Saigoh, K., Schiffmann, R., Asahara, H., Yamada, T., et al. (2006) Lamin B1 duplications cause autosomal dominant leukodystrophy, Nat. Genet., 38, 1114-1123, https://doi.org/10.1038/ng1872.
Hegele, R. A., Cao, H., Liu, D. M., Costain, G. A., Charlton-Menys, V., et al. (2006) Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy, Am. J. Hum. Genet., 79, 383-389, https://doi.org/10.1086/505885.
Cristofoli, F., Moss, T., Moore, H. W., Devriendt, K., Flanagan-Steet, H., et al. (2020) De novo variants in LMNB1 cause pronounced syndromic microcephaly and disruption of nuclear envelope integrity, Am. J. Hum. Genet., 107, 753-762, https://doi.org/10.1016/j.ajhg.2020.08.015.
Östlund, C., Chang, W., Gundersen, G. G., and Worman, H. J. (2019) Pathogenic mutations in genes encoding nuclear envelope proteins and defective nucleocytoplasmic connections, Exp. Biol. Med., 244, 1333-1344, https://doi.org/10.1177/1535370219862243.
Novelli, G., Muchir, A., Sangiuolo, F., Helbling-Leclerc, A., D’Apice, M. R., et al. (2002) Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding Lamin A/C, Am. J. Hum. Genet., 71, 426-431, https://doi.org/10.1086/341908.
Vigouroux, C., Magré, J., Vantyghem, M. C., Bourut, C., Lascols, O., et al. (2000) Lamin A/C gene: Sex-determined expression of mutations in Dunnigan-type familial partial lipodystrophy and absence of coding mutations in congenital and acquired generalized lipoatrophy, Diabetes, 49, 1958-1962, https://doi.org/10.2337/diabetes.49.11.1958.
Caux, F., Dubosclard, E., Lascols, O., Buendia, B., Chazouillères, O., et al. (2003) A new clinical condition linked to a novel mutation in lamins a and c with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy, J. Clin. Endocrinol. Metab., 88, 1006-1013, https://doi.org/10.1210/jc.2002-021506.
Fatkin, D., MacRae, C., Sasaki, T., Wolff, M. R., Porcu, M., et al. (1999) Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease, N. Engl. J. Med., 341, 1715-1724, https://doi.org/10.1056/NEJM199912023412302.
Hamadouche, T., Poitelon, Y., Genin, E., Chaouch, M., Tazir, M., et al. (2008) Founder effect and estimation of the age of the c.892C>T (p.Arg298Cys) mutation in LMNA associated to Charcot–Marie–Tooth subtype CMT2B1 in families from North Western Africa, Ann. Hum. Genet., 72, 590-597, https://doi.org/10.1111/j.1469-1809.2008.00456.x.
Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., et al. (2003) Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome, Nature, 423, 293-298, https://doi.org/10.1038/nature01629.
De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., et al. (2003) Lamin A truncation in Hutchinson–Gilford progeria, Science, 300, 2055, https://doi.org/10.1126/science.1084125.
Agarwal, A. K. (2003) Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia, Hum. Mol. Genet., 12, 1995-2001, https://doi.org/10.1093/hmg/ddg213.
Smigiel, R., Jakubiak, A., Esteves-Vieira, V., Szela, K., Halon, A., et al. (2010) Novel frameshifting mutations of the ZMPSTE24 gene in two siblings affected with restrictive dermopathy and review of the mutations described in the literature, Am. J. Med. Genet. Part A, 152, 447-452, https://doi.org/10.1002/ajmg.a.33221.
Navarro, C.L., Cau, P., and Lévy, N. (2006) Molecular bases of progeroid syndromes, Hum. Mol. Genet., 15, R151-61, https://doi.org/10.1093/hmg/ddl214.
Chong, J. X., Ouwenga, R., Anderson, R. L., Waggoner, D. J., and Ober, C. (2012) A population-based study of autosomal-recessive disease-causing mutations in a founder population, Am. J. Hum. Genet., 91, 608-620, https://doi.org/10.1016/j.ajhg.2012.08.007.
Khanna, P., Opitz, J. M., and Gilbert-Barness, E. (2008) Restrictive dermopathy: report and review, Fetal Pediatr. Pathol., 27, 105-118, https://doi.org/10.1080/15513810802077586.
Goldman, R. D., Shumaker, D. K., Erdos, M. R., Eriksson, M., Goldman, A. E., et al. (2004) Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome, Proc. Natl. Acad. Sci. USA, 101, 8963-8968, https://doi.org/10.1073/pnas.0402943101.
Scaffidi, P., and Misteli, T. (2006) Lamin A-dependent nuclear defects in human aging, Science, 312, 1059-1063, https://doi.org/10.1126/science.1127168.
Davidson, P. M., and Lammerding, J. (2014) Broken nuclei – lamins, nuclear mechanics, and disease, Trends Cell Biol., 24, 247-56, https://doi.org/10.1016/j.tcb.2013.11.004.
Worman, H. J., and Schirmer, E. C. (2015) Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr. Opin. Cell Biol., 34, 101-112, https://doi.org/10.1016/j.ceb.2015.06.003.
Osmanagic-Myers, S., and Foisner, R. (2019) The structural and gene expression hypotheses in laminopathic diseases – not so different after all, Mol. Biol. Cell, 30, 1786-1790, https://doi.org/10.1091/mbc.E18-10-0672.
Lutz, R. J., Trujillo, M. A., Denham, K. S., Wenger, L., and Sinensky, M. (1992) Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina, Proc. Natl. Acad. Sci. USA, 89, 3000-3004, https://doi.org/10.1073/pnas.89.7.3000.
Black, S. D. (1992) Development of hydrophobicity parameters for prenylated proteins, Biochem. Biophys. Res. Commun., 186, 1437-1442, https://doi.org/10.1016/S0006-291X(05)81567-0.
Hu, X.-T., Song, H.-C., Yu, H., Wu, Z.-C., Liu, X.-G., and Chen, W.-C. (2020) Overexpression of progerin results in impaired proliferation and invasion of non-small cell lung cancer cells, Onco. Targets. Ther., 13, 2629-2642, https://doi.org/10.2147/OTT.S237016.
Booth-Gauthier, E. A., Du, V., Ghibaudo, M., Rape, A. D., Dahl, K. N., and Ladoux, B. (2013) Hutchinson–Gilford progeria syndrome alters nuclear shape and reduces cell motility in three dimensional model substrates, Integr. Biol., 5, 569, https://doi.org/10.1039/c3ib20231c.
Ovsiannikova, N., Lavrushkina, S., Yudina, A., Strelkova, O., Zhironkina, O., and Kireev, I. (2019) The role of the nuclear lamina in cell migration: the connection with aging and metastasis, Biopolym. Cell, 35, 227-228, https://doi.org/10.7124/bc.0009F2.
De Vos, W. H., Houben, F., Kamps, M., Malhas, A., Verheyen, F., et al. (2011) Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies, Hum. Mol. Genet., 20, 4175-4186, https://doi.org/10.1093/hmg/ddr344.
Hatch, E. M., and Hetzer, M. W. (2016) Nuclear envelope rupture is induced by actin-based nucleus confinement, J. Cell Biol., 215, 27-36, https://doi.org/10.1083/jcb.201603053.
Cho, S., Abbas, A., Irianto, J., Ivanovska, I. L., Xia, Y., et al. (2018) Progerin phosphorylation in interphase is lower and less mechanosensitive than lamin-A,C in iPS-derived mesenchymal stem cells, Nucleus, 9, 235-250, https://doi.org/10.1080/19491034.2018.1460185.
Gordon, L. B., Rothman, F. G., López-Otín, C., and Misteli, T. (2014) Progeria: a paradigm for translational medicine, Cell, 156, 400-407, https://doi.org/10.1016/j.cell.2013.12.028.
Pfeifer, C. R., Xia, Y., Zhu, K., Liu, D., Irianto, J., et al. (2018) Constricted migration increases DNA damage and independently represses cell cycle, Mol. Biol. Cell, 29, 1948-1962, https://doi.org/10.1091/mbc.E18-02-0079.
Fernandez, P., Scaffidi, P., Markert, E., Lee, J.-H., Rane, S., and Misteli, T. (2014) Transformation resistance in a premature aging disorder identifies a tumor-protective function of BRD4, Cell Rep., 9, 248-260, https://doi.org/10.1016/j.celrep.2014.08.069.
Clarke, S. G. (2007) HIV protease inhibitors and nuclear lamin processing: Getting the right bells and whistles, Proc. Natl. Acad. Sci. USA, 104, 13857-13858, https://doi.org/10.1073/pnas.0706529104.
Coffinier, C., Hudon, S. E., Lee, R., Farber, E. A., Nobumori, C., et al. (2008) A Potent HIV protease inhibitor, darunavir, does not inhibit ZMPSTE24 or lead to an accumulation of farnesyl-prelamin A in cells, J. Biol. Chem., 283, 9797-9804, https://doi.org/10.1074/jbc.M709629200.
Liu, Q., Kim, D. I., Syme, J., LuValle, P., Burke, B., and Roux, K. J. (2010) Dynamics of lamin-A processing following precursor accumulation, PLoS One, 5, e10874, https://doi.org/10.1371/journal.pone.0010874.
Acknowledgments
The authors express their gratitude to E. D. Ryumina for her technical help in preparation of the text.
Funding
This work was financially supported by the Russian Foundation for Basic Research (project no. 19-315-90069) and by the Russian Science Foundation (project no. 17-15-01290).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ovsiannikova, N.L., Lavrushkina, S.V., Ivanova, A.V. et al. Lamin A as a Determinant of Mechanical Properties of the Cell Nucleus in Health and Disease. Biochemistry Moscow 86, 1288–1300 (2021). https://doi.org/10.1134/S0006297921100102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921100102