Abstract—
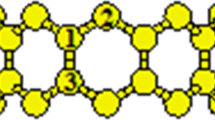
Quantum-mechanical models for the formation of metal–carbon complexes of Co, Ni, Cu, and Zn ions with С60 fullerene molecules and single-wall С48 carbon nanotubes (SWCNTs) are proposed. The results of calculations show that, in aqueous solutions of electrolytes, Co, Ni, Cu, and Zn ions can be adsorbed into the С60 fullerene and С48 SWCNT surfaces with the formation of stable carbon-nanomaterial—metal (CNM—M) complexes; in this case, the minimum energy of the С60–М complex for Co and Cu ions corresponds to the position above the С6 cell center; for a Ni ion, above the single С–С bond in the С6 cell; and for a Zn ion, above the C atom. The optimized states of the С48–М complexes correspond to the position of metal ions above the С6 cell center.

Similar content being viewed by others
REFERENCES
C. Gheorghies, D. E. Rusu, A. Bund, S. Condurache-Bota, and L. P. Georgescu, Appl Nanosci. 4, 1021 (2014). https://doi.org/10.1007/s13204-013-0285-y
G. K. Burkat, T. Fujimura, V. Yu. Dolmatov, E. A. Orlova, and M. V. Veretennikova, Diamond Relat. Mater. 14, 1761 (2005). https://doi.org/10.1016/j.diamond.2005.08.004
V. P. Isakov, A. I. Lyamkin, D. N. Nikitin, A. S. Shalimova, and A. V. Solntsev, Prot. Met. Phys. Chem. Surf. 46, 578 (2010). https://doi.org/10.1134/S2070205110050138
Liping Wang, Yan Gao, Qunji Xue, Huiwen Liu, and Tao Xu, Mater. Sci. Eng., A 390, 313 (2005). https://doi.org/10.1016/j.msea.2004.08.033
Zabludovs’kii V. O., Dudkina V. V., Shtapenko E. P., Nauka Progr. Transp. Vestn. Dnepropetr. Nats. Univ. im.V. Lazaryana 47 (5), 70 (2013). https://doi.org/10.15802/stp2013/17968
V. V. Dudkina, V. A. Zabludovskii, and E. F. Shtapenko, Metallofiz. Noveishie Tekhnol. 37, 713 (2015). https://doi.org/10.15407/mfint.37.05.0713
V. V. Titarenko and V. A. Zabludovskii, Metallofiz. Noveishie Tekhnol. 38, 519 (2016). Doi https://doi.org/10.15407/mfint.38.04.0519
V. V. Tytarenko, V. A. Zabludovsky, E. Ph. Shtapenko, and I. V. Tytarenko, Galvanotechnik, No. 4, 648 (2019).
G. A. Chiganova and L. E. Mordvinova, Inorg. Mater. 47, 717 (2011). https://doi.org/10.1134/S0020168511070089
Hiroshi Matsubara, Yoshihiro Abe, Yoshiyuki Chiba, Hiroshi Nishiyama, Nobuo Saito, Kazunori Hodouchi, and Yasunobu Inou, Acta Electrochim. 252, 3047 (2007). https://doi.org/10.1016/j.electacta.2006.09.043
Sam Zhang, Deen Sun, Yongqing Fu, and Hejun Du, Surf. Coat. Technol. 167, 113 (2003). https://doi.org/10.1016/S0257-8972(02)00903-9
J. G. Hou, Xiang Li, Haiqian Wang, and Bing Wang, J. Phys. Chem. Solids 61, 995 (2000). https://doi.org/10.1016/S0022-3697(99)00349-2
H. Valencia, A. Gil, and G. Frapper, J. Phys. Chem. C 114, 14141 (2010).
A. Larsson, D. Simon, J. C. Elliott, G. J. Repp, G. Meyer, and R. Allenspach, Phys. Rev. B: Condens. Matter Mater. Phys. 77, 115434 (2008). Rev B.77.115434https://doi.org/10.1103/Phys
E. F. Shtapenko, V. A. Zabludovskii, and E. O. Voronkov, Poverkhn.: Rentgenovskie, Sinkhrotronnye Neitr. Issled., No. 12, 95 (2010).
W. Koch and M. C. Holthausen, Chemists Guide to Density Functional Theory (Wiley, New York, 2001).
N. Lopez, N. Almora-Barrios, and G. Carchini, Catal. Sci. Technol. 2, 2405 (2012). https://doi.org/10.1039/C2CY20384G
T. C. Allison and Y. Y. Tong, Phys. Chem. Chem. Phys. 13, 12858 (2011). https://doi.org/10.1039/C1CP20376B
G. Schreckenbach, P. J. Hay, and R. L. Martin, Inorg. Chem. 37, 4442 (1998). https://doi.org/10.1002/(SICI)1096-987X(19990115)20:1<70::AID-JCC9>3.0.CO;2-F
B. Miehlich, A. Savin, H. Stoll, and H. Preuss, Chem. Phys. Lett. 157, 200 (1989). https://doi.org/10.1016/0009-2614(89)87234-3
D. A. Keire, Hee Jans Yun, Lin Li, et al., Inorg. Chem. 40, 4310 (2001). https://doi.org/10.1021/ic0010297
J. Andzelm and J. Labanowski, Density Functional Methods in Chemistry (Springer, Heidelberg, 1991). https://doi.org/10.1007/978-1-4612-3136-3
S. Grimme, A. J. Grimme, S. Ehrlich, and H. Krieg, J. Phys. Chem. 132, 154104 (2010). https://doi.org/10.1063/1.3382344
S. Grimme, EhrlichS. Grimme, and L. Goerigk, J. Comput. Chem. 32, 1456 (2011). https://doi.org/10.1002/jcc.21759
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev. 105, 2999 (2005). https://doi.org/10.1021/cr9904009
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Caimni, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. Al’Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision C.02 (Gaussian, Wallingford, 2004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by L. Kulman
Rights and permissions
About this article
Cite this article
Tytarenko, V.V., Shtapenko, E.P., Voronkov, E.O. et al. Quantum-Mechanical Modeling of the Interaction between Carbon Nanostructures and Metal Ions. J. Surf. Investig. 15, 866–871 (2021). https://doi.org/10.1134/S102745102104039X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102745102104039X