Abstract
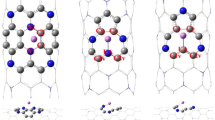
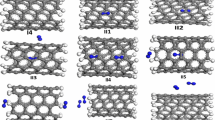
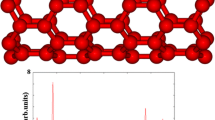
Single-atom confinement inside carbon nanotubes has attracted much attention in many fields. This class of materials may not only serve as a catalyst but also as a support material for certain reactions. In this paper, we have studied the single-walled carbon nanotubes (SWCNT), single vacancy defect (SV), and Stone–Wales defect (SW) carbon nanotubes with Fe, Co, and Ni atom by both inside and outside adsorption structures in density function theory (DFT). Our results reveal that the binding abilities of atomic Fe, Co, Ni onto the internal and external surfaces of the SWCNT, SV, and SW are in following orders by metals: Ni > Co > Fe. The adsorption energies of SV toward Fe, Co, and Ni are more stable than those of SWCNT and SW, which can be attributed to the three active carbon sites created by a C atom removing, while the SWCNT and SW demonstrate similar adsorption energy due to the similar structure. Generally, the stability of external adsorption structures is stronger than those of internal adsorption structures, but as for the SW, the stability of internal and external adsorption structures is close, which means that the defects have improved the confinement of carbon nanotubes to M (M = Fe, Co, Ni).




Similar content being viewed by others
REFERENCES
Y. Peng, B. Lu, and S. Chen, Adv. Mater. Adv. Mater. 30, 1801995 (2018).
Q. Wang, D. Zhang, Y. Chen, et al., ACS Sustain. Chem. Eng. 7, 6430 (2019).
M. M. Millet, G. Algara-Siller, S. Wrabetz, et al., J. Am. Chem. Soc. 141, 2451 (2019).
J. Amsler, B. B. Sarma, G. Agostini, et al., J. Am. Chem. Soc. 142, 5087 (2020).
K. Yuan, S. Feng, F. Zhang, et al., Ind. Eng. Chem. Res. 59, 19680 (2020).
C. Shao, C. Rui, J. Liu, et al., Ind. Eng. Chem. Res. 59, 19593 (2020).
G. S. Tulevski and A. L. Falk, Adv. Funct. Mater. 30, 1909448 (2020).
J. Banerjee and K. Dutta, Polym. Compos. 40, 4473 (2019).
X. Lu, Z. Chen, and P. V. R. Schleyer, J. Am. Chem. Soc. 127, 20 (2005).
T. C. Dinadayalane, J. S. Murray, M. C. Concha, et al., J. Chem. Theory Comput. 6, 1351 (2010).
T. C. Dinadayalane and J. Leszczynski, Chem. Phys. Lett. 434, 86 (2007).
A. L. Arokiyanathan, N. Panjulingam, and S. Lakshmipathi, J. Phys. Chem. C 124, 7229 (2020).
L. Li, S. Reich, and J. Roberton, Phys. Rev. B 72, 184109 (2005).
W. Wu, W. Zhang, Y. Long, et al., Mol. Catal. 497, 111226 (2020).
N. Gyanchani, S. Pawar, P. Maheshwary, et al., Mater. Sci. Eng. B 261, 114772 (2020).
M. Vichel, M. Busch, and K. Laasonen, ChemCatChem 12, 1436 (2020).
X. Zhang, S. Zhang, Y. Yang, et al., Adv. Mater. 32, 1906905 (2020).
P. A. Loginov, U. A. Zhassay, M. Y. Bychkova, et al., Int. J. Refract. Met. Hard Mater. 92, 105289 (2020).
L. Wu, Y. Lu, W. Shao, et al., Adv. Mater. Interfaces 7, 2000736 (2020).
X. Chen, F. Ge, J. Chang, et al., Int. J. Energ. Res. 43, 7375 (2019).
G. Mei, L. Cui, Z. Dou, et al., Electrochim. Acta 358, 136918 (2020).
Q. Liu, H. Zhang, J. Xu, et al., Inorg. Chem. 57, 15610 (2018).
X. J. Liu, Y. D. Sun, X. Yin, et al., Energy Fuel 34 (2020).
J. Liu, J. Lan, L. Yang, et al., ACS Sustain. Chem. Eng. 7, 6541 (2019).
L. Cao, Y. Shao, H. Pan, et al., J. Phys. Chem. C 124, 11301 (2019).
M. A. Kazakova, D. M. Morales, C. Andronescu, et al., Catal. Today 357, 259 (2020).
J. Yi, X. Liu, P. Liang, et al., Organometallics 38, 1186 (2019).
S. Guo, X. Pan, H. Gao, Z. Yang, et al., Chem. Eur. J. 16, 5379 (2020).
H. Friedrich, S. Guo, P. E. de Jongh, et al., ChemSusChem 4, 975 (2011).
J. Chen, W. Zhou, Z. Zhu, et al., Carbon 49, 2022 (2011).
X. Lin, X. Wang, L. Li, et al., ACS Sustain. Chem. Eng. 5, 9709 (2017).
F. Pan, B. Li, E. Sarnello, et al., ACS Nano 14, 5506 (2020).
Q. Fu, W. Li, Y. Yao, et al., Science (Washington, DC, U. S.) 328 (2010).
Q. Fu, F. Yang, and X. Bao, Acc. Chem. Res. 46, 1692 (2013).
W. Chen, X. Pan, and X. Bao, J. Am. Chem. Soc. 129, 7421 (2007).
Z. Yang, J. Qian, A. Yu, et al., Proc. Natl. Acad. Sci. U. S. A. 116, 6659 (2019).
H. Zhang, J. Wang, Z. Zhao, et al., Green Chem. 20, 3521 (2018).
P. Su, M. Zhou, G. Ren, et al., J. Mater. Chem. A 7, 24408 (2019).
B. Delley, J. Chem. Phys. 92, 508 (1990).
B. Delley, J. Chem. Phys. 113, 7756 (2000).
Y. Tang, Z. Yang, and X. Dai, Phys. Chem. Chem. Phys. 14, 16566 (2012).
J. Zhao, Y. Chen, and H. Fu, Theor. Chem. Acc. 131, 1 (2012).
M. Piacenza and S. Grimme, J. Comput. Chem. 25, 83 (2004).
S. Grimme, J. Comput. Chem. 27, 1787 (2006).
ACKNOWLEDGMENTS
This article was funded by the Natural Science Foundation of Gansu (no. 20JR5RA199) and National Natural Science Foundation (no. 21865007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wang, QY., Nan, G., Chen, YY. et al. Theoretical Study on the Structures of Single-Atom M (M = Fe, Co, and Ni) Adsorption Outside and Inside the Defect Carbon Nanotubes. Russ. J. Phys. Chem. 96 (Suppl 1), S145–S152 (2022). https://doi.org/10.1134/S0036024422140254
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422140254