Abstract
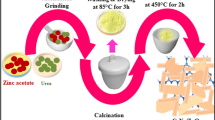
Zinc ferrite nanoparticles are synthesized by a microwave assisted polyacrylamide gel route. The influence of different Zn salts includes zinc nitrate, zinc sulfate, zinc chloride, zinc acetate on the crystal structure, surface morphologies, optical properties, magnetic properties, and photocatalytic activity of the ZnFe2O4 nanoparticles were systematically studied. The ZnFe2O4 nanoparticles prepared using zinc nitrate have cubic spinel structure and exhibited good size uniformity and regularity. The absorption edge of ZnFe2O4 nanoparticles prepared using zinc nitrate as Zn salt shifted to a higher energy compared with that of ZnFe2O4 nanoparticles prepared by other Zn salts. The magnetic susceptibility indicates that the blocking temperature (TB) decreases from 94 to 35 K with Zn salt change from zinc nitrate to zinc sulfate due to the size effect. Interesting, zinc nitrate is used as Zn salt improves the photocatalytic activity for the degradation of rhodamine B (RhB) dye wastewater of ZnFe2O4 nanoparticles significantly due to introduced the surface species of OH– to the ZnFe2O4 nanopartciles. The recycling experiment indicates that the ZnFe2O4 nanopartciles have a high stability. The photocatalytic mechanism of ZnFe2O4 nanopartciles have been systematically studied on the basis of the photocatalytic experiment and electrochemical test.











Similar content being viewed by others
REFERENCES
R. C. Che, L. M. Peng, X. F. Duan, Q. Che, and X. L. Liang, Adv. Mater. 16, 401 (2004).
A. Moser, K. Takano, D. T. Margulies, M. Albrecht, Y. Sonobe, Y. Ikeda, S. Sun, and E. E. Fullerton, J. Phys. D: Appl. Phys. 35, R157 (2002).
J. M. Bai and J. P. Wang, Appl. Phys. Lett. 87, 152502 (2005).
S. W. Cao, Y. J. Zhu, G. F. Cheng, and Y. H. Huang, J. Hazard. Mater. 171, 431 (2009).
K. Raj and R. Moskowitz, J. Magn. Magn. Mater. 85, 233 (1990).
R. Dom, A. S. Chary, R. Subasri, N. Y. Hebalkar, and P. H. Borse, Int. J. Energ. Res. 39, 1378 (2015).
T. Tabari, D. Singh, and S. S. Jamali, J. Environ. Chem. Eng. 5, 931 (2017).
F. Mueller, D. Bresser, E. Paillard, M. Winter, and S. Passerini, J. Power Sources 236, 87 (2013).
J. Li, A. R. Wang, Y. Q. Lin, X. D. Liu, J. Fu, and L. H. Lin, J. Magn. Magn. Mater. 330, 96 (2013).
F. Li, Y. Q. Qian, and A. Stein, Chem. Mater. 22, 3226 (2010).
V. Blanco-Gutierrez, E. Urones-Garrote, M. J. Torralvo-Fernandez, and R. Saez-Puche, Chem. Mater. 22, 6130 (2010).
C. W. Yao, Q. S. Zeng, G. F. Goya, T. Torres, J. F. Liu, H. P. Wu, M. Y. Ge, Y. W. Zeng, Y. W. Wang, and J. Z. Jiang, J. Phys. Chem. C 111, 12274 (2007).
J. Feng, Z. Zhang, M. Gao, M. Gu, J. Wang, W. Zeng, Y. Z. Lv, Y. M. Ren, and Z. Fan, Mater. Chem. Phys. 223, 758 (2019).
F. Li, H. Wang, L. Wang, and J. Wang, J. Magn. Magn. Mater. 309, 295 (2007).
U. Kurtan, H. Erdemi, A. Baykal, and H. Güngünes, Ceram. Int. 42, 13350 (2016).
P. A. Vinosha, L. A. Mely, J. E. Jeronsia, S. Krishnan, and S. J. Das, Optik 134, 99 (2017).
A. F. S. Abu-Hani, S. T. Mahmoud, F. Awwad, and A. I. Ayesh, Sens. Actuators, B 241, 1179 (2017).
C. Wang, Y. Li, Y. Ruan, J. Jiang, and Q. H. Wu, Mater. Today Energ. 3, 1 (2017).
M. Amir, H. Gungunes, A. Baykal, M. A. Almessiere, H. Sözeri, I. Ercan, M. Sertkol, S. Asiri, and A. Manikandan, J. Supercond. Nov. Magn. 31, 3347 (2018).
Z. Xing, Z. Ju, J. Yang, H. Xu, and Y. Qian, Nano Res. 5, 477 (2012).
Y. Köseoglu, A. Baykal, M. S. Toprak, F. Gözüak, A. C. Basaran, and B. Aktas, J. Alloys Compd. 462, 209 (2008).
S. F. Wang, X. T. Zu, G. Z. Sun, D. M. Li, C. D. He, X. Xiang, W. Liu, S. B. Han, and S. Li, Ceram. Int. 42, 19133 (2016).
S. F. Wang, Q. Li, X. T. Zu, X. Xiang, W. Liu, and S. Li, J. Magn. Magn. Mater. 419, 464 (2016).
D. F. Zhao, H. Yang, R. S. Li, J. Y. Ma, and W. J. Feng, Mater. Res. Innov. 18, 519 (2014).
W. P. Wang, H. Yang, T. Xian, and J. L. Jiang, Mater. Trans. 53, 1586 (2012).
F. Hu, S. Zhao, and X. Yin, J. Mater. Sci. Mater. Electron. 29, 16747 (2018).
A. Bigham, F. Foroughi, M. Motamedi, and M. Rafienia, Ceram. Int. 44, 11798 (2018).
M. L. Aparna, A. N. Grace, P. Sathyanarayanan, and N. K. Sahu, J. Alloys Compd. 745, 385 (2018).
X. F. She and Z. Zhang, Nanoscale Res. Lett. 12, 211 (2017).
Z. W. Wang, D. Schiferl, Y. S. Zhao, and C. O’Neill, J. Phys. Chem. Solids 64, 2517 (2003).
Z. Cvejic, S. Rakic, A. Kremenovic, B. Antic, C. Jovalekic, and P. Colomban, Solid State Sci. 8, 908 (2006).
S. Urcia-Romero, O. Perales-Pérez, and G. Gutiérrez, J. Appl. Phys. 107, 09A508 (2010).
G. Shemer, E. Tirosh, T. Livneh, and G. Markovich, J. Phys. Chem. C 111, 14334 (2007).
W. Liu, Y. Chan, J. Cai, C. Leung, C. Mak, K. Wong, F. Zhang, X. Wu, and X. D. Qi, J. Appl. Phys. 112, 104306 (2012).
X. Zhao, W. Wang, Y. Zhang, S. Wu, F. Li, and J. P. Liu, Chem. Eng. J. 250, 164 (2014).
N. Romcevic, R. Kostic, M. Romcevic, B. Hadzic, I. Kuryliszyn-Kudelska, W. Dobrowolski, and D. Sibera, Acta Phys. Polon. A 114, 1323 (2008).
A. N. Ay, B. Zümreoglu-Karan, A. Temel, and V. Rives, Inorg. Chem. 48, 8871 (2009).
A. Silambarasu, A. Manikandan, and K. Balakrishnan, J. Supercond. Nov. Magn. 30, 2631 (2017).
P. Jeevanandam, Y. Koltypin, and A. Gedanken, Mater. Sci. Eng. B 90, 125 (2002).
S. H. Xu, D. L. Feng, and W. F. Shanggua, J. Phys. Chem. C 113, 2463 (2009).
G. L. Fan, Z. J. Gu, L. Yang, and F. Li, Chem. Eng. J. 155, 534 (2009).
X. Y. Li, Y. Hou, Q. D. Zhao, and L. Z. Wang, J. Colloid Interf. Sci. 358, 102 (2011).
K. Woo, H. J. Lee, P. Ahn and Y. S. Park, Adv. Mater. 15, 1761 (2010).
Y. S. Wang, A. Muramatsu, and T. Sugimoto, Colloid Surf. A 134, 281 (1998).
A. Kaschner, U. Haboeck, M. Strassburg, M. Strassburg, G. Kaczmarczyk, A. Hoffmann, C. Thomsen, A. Zeuner, H. R. Alves, D. M. Hofmann, and B. K. Meyer, Appl. Phys. Lett. 80, 1909 (2002).
G. Xiong, U. Pal, J. G. Serrano, K. B. Ucer, and R. T. Williams, Phys. Status Solidi C 3, 3577 (2006).
X. Guo, H. J. Zhu, M. S. Si, C. J. Jiang, D. S. Xue, Z. H. Zhang, and Q. Li, J. Phys. Chem. C 119, 30145 (2014).
G. K. Zhang, M. Li, S. J. Yu, S. M. Zhang, B. B. Huang, and J. G. Yu, J. Colloid Interface Sci. 345, 467 (2010).
Z. H. Yuan, W. You, J. H. Jia, and L. Zhang, Chin. Phys. Lett. 15, 535 (1998).
L. J. Han, X. Zhou, L. N. Wan, Y. F. Deng, and S. Z. Zhan, J. Environ. Chem. Eng. 2, 123 (2014).
N. Kislov, S. S. Srinivasan, Yu. Emirov, and E. K. Stefanakos, Mater. Sci. Eng. B 153, 70 (2008).
H. Fu, S. Zhang, T. Xu, Y. Zhu, and J. Chen, Environ. Sci. Technol. 42, 2085 (2008).
Z. Cui, H. Yang, and X. Zhao, Mater. Sci. Eng. B 229, 160 (2018).
X. X. Wang, Y. Li, M. C. Liu, and L. B. Kong, Ionics 24, 363 (2018).
X. Zhao, H. Yang, Z. Cui, R. Li, and W. Feng, Mater. Technol. 32, 870 (2017).
S. Horikoshi, A. Saitou, H. Hidaka, and N. Serpone, Environ. Sci. Technol. 37, 5813 (2003).
Z. X. Chen, D. Z. Li, W. J. Zhang, Y. Shao, T. W. Chen, M. Sun, and X. Z. Fu, J. Phys. Chem. C 113, 4433 (2009).
S. R. Morrison, Electrochemistry at Semiconductor and Oxidized Metal Electrodes (Plenum, New York, NY, 1980).
R. Dom, R. Subasri, K. Radha, and P. H. Borse, Solid State Commun. 151, 470 (2011).
ACKNOWLEDGMENTS
This work was financially supported by National Natural Science Foundation of China (51678409).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You Wang, Yang, L., Zhang, Y. et al. Magnetic, Optical Properties, and Photocatalytic Activity of the ZnFe2O4 Nanoparticles for the Degradation of the RhB Dye in Wastewater: Effects of Metal Salt and Surface Morphology. Russ. J. Phys. Chem. 93, 2771–2781 (2019). https://doi.org/10.1134/S0036024419130314
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419130314