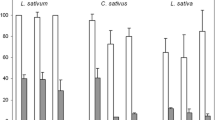
Abstract
Indole derivatives were synthetized based on the Fischer indole methodology using different phenyl hydrazine hydrochlorides and either cyclohexanone or 2-butanone. The pre- and post-emergent herbicidal activities were evaluated against Ipomoea grandifolia. A carbazole, 6-chloro-2,3,4,9-tetrahydro-1H-carbazole (3b), decreased the PIabs parameter by 32% and increased the cross-section related parameters, indicating the inactivation of the reaction center on photosystem II. Compound 3b acts as a post-emergent herbicide prototype since dry biomass was reduced by 50%, corroborating the fluorescence results. Comparing instead with a germination experiment, 2,3,4,9-tetrahydro-1H-carbazole (3a)was found to be the most effective agent, inhibiting seed germination by 22% and decreasing root length by 50%. The tetrahydrocarbazoles showed better results than indole derivatives potentially due to the presence of methylene groups at structures, which increase the compounds' lipophilicity and may facilitate their access to the plant. In addition, electron withdrawing groups on the aromatic ring were found to correlate with increased herbicide activity. Further optimization of this series towards the development of herbicides is ongoing.
Similar content being viewed by others
References
J. R. Vyvyan, Allelochemicals as leads for new herbicides and agrochemicals, Tetrahedron, 2002, 58, 1631–1646.
F. E. Dayan, C. L. Cantrell and S. O. Duke, Natural products in crop protection, Bioorg. Med. Chem., 2009, 17, 4022–4034.
I. Heap, Pest Manage. Sci., 2014, 70, 1306–1315.
L. C. A. Barbosa, A. J. Demuner, E. S. De Alvarenga, A. Oliveira, B. King-Diaz and B. Lotina-Hennsen, Phytogrowth- and photosynthesis-inhibiting properties of nostoclide analogues, Pest Manage. Sci., 2006, 62, 214–222.
M. I. Aguilar, M. G. Romero, M. I. Chávez, B. King-Díaz and B. Lotina-Hennsen, Biflavonoids isolated from Selaginella lepidophylla inhibit photosynthesis in spinach chloroplasts, J. Agric. Food Chem., 2008, 56, 6994–7000.
D. Torres-Romero, B. King-Díaz, I. A. Jiménez, B. Lotina-Hennsen and I. L. Bazzocchi, Sesquiterpenes from Celastrus vulcanicola as photosynthetic inhibitors, J. Nat. Prod., 2008, 71, 1331–1335.
T. A. M. Veiga, B. King-Díaz, A. S. F. Marques, O. M. Sampaio, P. C. Vieira, M. F. G. F. Da Silva and B. Lotina-Hennsen, Furoquinoline alkaloids isolated from Balfourodendron riedelianum as photosynthetic inhibitors in spinach chloroplasts, J. Photochem. Photobiol., B, 2013, 120, 36–43.
J. O. S. Varejao, L. C. A. Barbosa, E. V. V. Varejao, C. R. A. Maltha, B. King-Díaz and B. Lotina-Hennsen, Cyclopent-4-ene-1,3-diones: A new class of herbicides acting as potent photosynthesis inhibitors, J. Agric. Food Chem., 2014, 62, 5772–5780.
L. Semelkova, K. Konecna, P. Paterova, V. Kubicek, J. Kunes, L. Novakova, J. Marek, L. Naesens, M. Pesko, K. Kralova, M. Dolezal and J. Zitko, Synthesis and biological evaluation of N-alkyl-3-(alkylamino)-pyrazine-2-carboxamides, Molecules, 2015, 20, 8687–8711.
O. M. Sampaio, M. M. C. de Lima, T. A. M. Veiga, B. King-Díaz, M. F. G. F. Da Silva and B. Lotina-Hennsen, Evaluation of antidesmone alkaloid as a photosynthesis inhibitor, Pestic. Biochem. Physiol., 2016, 134, 55–62.
D. Q. Yang, Y. L. Luo, W. H. Dong, Y. P. Yin, Y. Li and Z. L. Wang, Response of photosystem II performance and antioxidant enzyme activities in stay-green wheat to cytokinin, Photosynthetica, 2018, 56, 567–577.
M. Franic, V. Galie, M. Mazur and D. Šimie, Effects of excess cadmium in soil on JIP-test parameters, hydrogen peroxide content and antioxidant activity in two maize inbreds and their hybrid, Photosynthetica, 2018, 56, 660–669.
R. J. Strasser, M. Tsimilli-Michael and A. Srivastava, in Chlorophyll a Fluorescence, 2004, pp. 321–362.
H. X. Ding, K. K.-C. Liu, S. M. Sakya, A. C. Flick and C. J. O'Donnell, Synthetic approaches to the 2011 new drugs, Bioorg. Med. Chem., 2013, 21, 2795–2825.
T. D. Penning, Small-molecule PARP modulators-current status and future therapeutic potential., Curr. Opin. Drug Discovery Dev., 2010, 13, 577–586.
N. Charrier, B. Clarke, L. Cutler, E. Demont, C. Dingwall, R. Dunsdon, P. East, J. Hawkins, C. Howes, I. Hussain, P. Jeffrey, G. Maile, R. Matico, J. Mosley, A. Naylor, A. O'Brien, S. Redshaw, P. Rowland, V. Soleil, K. J. Smith, S. Sweitzer, P. Theobald, D. Vesey, D. S. Walter and G. Wayne, Second generation of hydroxyethylamine BACE-1 inhibitors: Optimizing potency and oral bioavailability, J. Med. Chem., 2008, 51, 3313–3317.
V. Sharma, P. Kumar and D. Pathaka, Biological importance of the indole nucleus in recent years: A comprehensive review, J. Heterocycl. Chem., 2010, 47, 491–502.
T. V. Sravanthi and S. L. Manju, Eur. J. Pharm. Sci., 2016, 91, 1–10.
H. Zhang, Q. Wang, X. Ning, H. Hang, J. Ma, X. Yang, X. Lu, J. Zhang, Y. Li, C. Niu, H. Song, X. Wang and P. G. Wang, Synthesis and Biological Evaluations of a Series of Thaxtomin Analogues, J. Agric. Food Chem., 2015, 63, 3734–3741.
D. Sipaviciute, D. Tavgeniene, G. Krucaite, J. Grazulevicius, D. Volyniuk, B. Yao, Z. Xie, B. Zhang and S. Grigalevicius, Twin compounds of phenylethenyl substituted indole as efficient materials for electroluminescent devices, 2016, vol. 134.
M. Zhang, G. Qin, J. Liu, Z. Zhen, A. A. Fedorchuk, G. Lakshminarayana, A. A. Albassam, A. M. El-Naggar, K. Ozga and I. V. Kityk, Modification of indole by electronrich atoms and their application in novel electron donor materials, Chem. Phys. Lett., 2017, 681, 105–109.
S. Kotha, M. Saifuddin and V. R. Aswar, A diversity-oriented approach to indolocarbazoles via Fischer indolization and olefin metathesis: total synthesis of tjipanazole D and I, Org. Biomol. Chem., 2016, 14, 9868–9873.
T. Heinrich and H. Böttcher, A new synthesis of indole 5-car-boxylic acids and 6-hydroxy-indole-5- carboxylic acids in the preparation of an o-hydroxylated metabolite of vilazodone, Bioorg. Med. Chem. Lett., 2004, 14, 2681–2684.
D.-Q. Xu, J. Wu, S.-P. Luo, J.-X. Zhang, J.-Y. Wu, X.-H. Du and Z.-Y. Xu, Fischer indole synthesis catalyzed by novel SO3H-functionalized ionic liquids in water, Green Chem., 2009, 11, 1239.
S. Gore, S. Baskaran and B. König, Fischer indole synthesis in low melting mixtures, Org. Lett., 2012, 14, 4568–4571.
Z. G. Zhang, B. A. Haag, J. S. Li and P. Knochel, Efficient preparation of polyfunctional indoles via a zinc organometallic variation of the Fischer indole synthesis, Synthesis, 2011, 23–29.
A. R. Katritzky, G. W. Rewcastle and L. M. Vazquez de Miguel, Improved Syntheses of Substituted Carbazoles and Benzocarbazoles Via Lithiation of the (Dialkylamino) Methyl (Aminal) Derivatives, J. Org. Chem., 1988, 53, 794–799.
D. Deorha and S. Joshi, Notes- Cyclic Products from Hydrazines. I. Nitroindoles, Nitrotetrahydrocarbazoles, Nitroindolenines and Nitrotetrahydrocarbazolenines, J. Org. Chem., 1961, 26, 3527–3530.
Y. Li, T. Yan, K. Junge and M. Beller, Catalytic methylation of C-H bonds using CO2 and H2, Angew. Chem., Int. Ed., 2014, 53, 10476–10480.
M. Tursky, L. L. R. Lorentz-Petersen, L. B. Olsen and R. Madsen, Iridium- and ruthenium-catalysed synthesis of 2,3-disubstituted indoles from anilines and vicinal diols, Org. Biomol. Chem., 2010, 8, 5576.
M. Takemoto, Y. Iwakiri and K. Tanaka, Oxidative Cleavage Reaction of Substituted Indoles Catalyzed by Plant Cell Cultures, 2007, vol. 72.
T. Barf, F. Lehmann, K. Hammer, S. Haile, E. Axen, C. Medina, J. Uppenberg, S. Svensson, L. Rondahl and T. Lundbäck, N-Benzyl-indolo carboxylic acids: Design and synthesis of potent and selective adipocyte fatty-acid binding protein (A-FABP) inhibitors, Bioorg. Med. Chem. Lett., 2009, 19, 1745–1748.
P. E. Verkade and J. Lieste, Untersuchungen über indolderivate. X. Einige von 2,3-Dimethylindol abgeleitete Bz-Carbonsäuren, Recl. Trav. Chim. Pays-Bas, 1946, 65, 912–918.
M. R. Schmer, Q. Xue and J. R. Hendrickson, Salinity effects on perennial, warm-season (C 4) grass germination adapted to the northern Great Plains, Can. J. Plant Sci., 2012, 92, 873–881.
S. J. Steinmaus, T. S. Prather and J. S. Holt, Estimation of base temperatures for nine weed species, J. Exp. Bot., 2000, 51, 275–286.
R. J. Strasser, A. Srivastava and M. Tsimilli-Michael, The fluorescence transient as a tool to characterize and screen photosynthetic samples, Probing Photosynth. Mech. Regul. Adapt., 2000, 443–480.
O. M. Sampaio, M. F. das G. F. da Silva, T. A. M. Veiga, B. King-Díaz and B. Lotina-Hennsen, Química Nov., 2012, 35, 2115–2118.
L.-L. Meng, J.-F. Song, J. Wen, J. Zhang and J.-H. Wei, Effects of drought stress on fluorescence characteristics of photosystem II in leaves of Plectranthus scutellarioides, Photosynthetica, 2016, 54, 414–421.
A. Digrado, L. G. de la Motte, A. Bachy, A. Mozaffar, N. Schoon, F. Bussotti, C. Amelynck, A.-C. Dalcq, M.-L. Fauconnier, M. Aubinet, B. Heinesch, P. du Jardin and P. Delaplace, Decrease in the Photosynthetic Performance of Temperate Grassland Species Does Not Lead to a Decline in the Gross Primary Production of the Ecosystem, Front. Plant Sci., 2018, 9, 67.
E. G. Martinazzo, A. T. Perboni, P. V. De Oliveira, V. J. Bianchi and M. A. Bacarin, Photosynthetic activity in japanese plum under water deficit and flooding, Cienc. Rural, 2013, 43, 35–41.
V. D. Kreslavski, M. Brestic, S. K. Zharmukhamedov, V. Yu. Lyubimov, A. V. Lankin, A. Jajoo and S. I. Allakhverdiev, Mechanisms of inhibitory effects of polycyclic aromatic hydrocarbons in photosynthetic primary processes in pea leaves and thylakoid preparations, Plant Biol., 2017, 19, 683–688.
K. Zushi, S. Kajiwara and N. Matsuzoe, Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit, Sci. Hortic., 2012, 148, 39–46.
D. Pazuch, M. M. Trezzi, A. C. D. Guimaraes, M. V. J. Barancelli, R. Pasini and R. A. Vidal, Evolution of Natural Resistance to Glyphosate in Morning Glory Populations, Planta Daninha, 2017, 35,1–9.
P. J. Christoffoleti, A. Borges, M. Nicolai, S. J. P. Carvalho, R. F. López-Ovejero and P. A. Monquero, Planta Daninha, 2006, 24, 83–90.
A. G. Ferreira and M. E. A. Aquila, Alelopatia: uma área emergente da ecofisiologia, Rev. Bras. Fisiol. Veg., 2000, 12, 175–204.
C. M. Tice, Selecting the right compounds for screening: Does Lipinski’s rule of 5 for pharmaceuticals apply to agrochemicals?, Pest Manage. Sci., 2001, 57, 3–16.
A. D. Gribble, R. J. Ife, A. Shaw, D. McNair, C. E. Novelli, S. Bakewell, V. P. Shah, R. E. Dolle, P. H. Groot, N. Pearce, J. Yates, D. Tew, H. Boyd, S. Ashman, D. S. Eggleston, R. Curtis Haltiwanger and G. Okafo, ATP-citrate lyase as a target for hypolipidemic intervention. 2. Synthesis and evaluation of (3R*,5S*)-rn-substituted-3-carboxy-3,5-di-hydroxyalkanoic acids and their y-lactone prodrugs as inhibitors of the enzyme in vitro and in vivo, J. Med. Chem., 1998, 41, 3582–3595.
M. Roche, J. Bignon, J. D. Brion, A. Hamze and M. Alami, Tandem one-pot palladium-catalyzed coupling of hydrazones, haloindoles, and amines: Synthesis of amino-N-vinylindoles and their effect on human colon carcinoma cells, J. Org. Chem., 2014, 79, 7583–7592.
A. Walser, T. Flynn, C. Mason, H. Crowley, C. Maresca, B. Yaremko and M. O’Donnell, Triazolobenzo- and Triazolothienodiazepines as Potent Antagonists of Platelet Activating Factor, J. Med. Chem., 1991, 34, 1209–1221.
J. Timothy, M. Ibanez, A. C. Rijnbrand, A. J. Jackson, G. P. Mittapalli, F. Zhao, J. E. MacDonald and F. Wong-Staal, Hepatitis c virus entry inhibitors, WO 2008021745A3, 2008.
M. Ohashi, T. Shudo, K. Nishijima, T. Notsu, A. Kikuchi, K. Yanagibashi and H. Nishida, Pyridocarbazol derivate die einen cGMP-PDE inhibilierenden effekt haben, EP 0985671, 2001.
M. Ohashi, T. Shudo, K. Nishijima, T. Notsu, A. Kikuchi, K. Yanagibashi and H. Nishida, Pyridocarbazole derivatives having cgmp-pde inhibitory activity, US 6197768, 2001.
Acknowledgements
We gratefully acknowledge the FAPEMAT (grants 0217830/2017 and 0250685/2017), CNPq (grant 430089/2016-3) and UFMT for financial support and fellowships. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva Mendes, M.C., Fazolo, B.R., de Souza, J.M. et al. Synthesis and evaluation of indole derivatives as photosynthesis and plant growth inhibitors. Photochem Photobiol Sci 18, 1350–1358 (2019). https://doi.org/10.1039/c8pp00506k
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00506k