Abstract
Background
Obstructive nephropathy is a condition often caused by urinary tract obstruction either anatomical (e.g., tumors), mechanical (e.g., urolithiasis), or compression (e.g., pregnancy) and can progress to chronic kidney disease (CKD). Studies have shown sexual dimorphism in CKD, where males were found to have a more rapid decline in kidney function following kidney injury compared to age-matched females. Protocatechuic acid (PCA), an anti-oxidant and anti-inflammatory polyphenolic compound, has demonstrated promising effects in mitigating drug-induced kidney injuries. The current study aims to explore sexual dimorphism in kidney injury after unilateral ureteral obstruction (UUO) and assess whether PCA treatment can mitigate kidney injury in both sexes.
Methods
UUO was induced in 10–12 weeks old male and female C57BL/6J mice. Mice were categorized into four groups (n = 6–8/group); Sham, Sham plus PCA (100 mg/kg, I.P daily), UUO, and UUO plus PCA.
Results
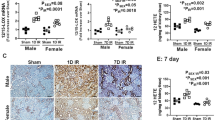
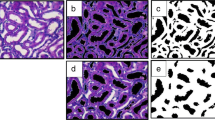
After 2 weeks of induction of UUO, markers of kidney oxidative stress (TBARs), inflammation (IL-1α and IL-6), tubular injury (neutrophil gelatinase-associated lipocalin, NGAL and urinary kidney injury molecule-1, KIM-1), fibrosis (Masson's trichrome staining, collagen IV expression, MMP-2 and MMP-9) and apoptosis (TUNEL+ cells, active caspase-1 and caspase-3) were significantly elevated in both males and females relative to their sham counterparts. Males exhibited significantly greater kidney oxidative stress, inflammation, fibrosis, and apoptosis after induction of UUO when compared to females. PCA treatment significantly attenuated UUO-induced kidney injury, inflammation, fibrosis, and apoptosis in both sexes.
Conclusion
Our findings suggest a differential gender response to UUO-induced kidney injury with males being more sensitive to UUO-induced kidney inflammation, fibrosis, and apoptosis than age-matched females. Importantly, PCA treatment reduced UUO-induced kidney injury in a sex-independent manner which might be attributed to its anti-oxidant, anti-inflammatory, anti-fibrotic, and anti-apoptotic properties.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Luyckx VA, Al-Aly Z, Bello AK, Bellorin-Font E, Carlini RG, Fabian J, et al. Sustainable Development Goals relevant to kidney health: an update on progress. Nat Rev Nephrol. 2021;17(1):15–32.
Chang PY, Chien LN, Lin YF, Wu MS, Chiu WT, Chiou HY. Risk factors of gender for renal progression in patients with early chronic kidney disease. Medicine (Baltimore). 2016;95(30): e4203.
Chesnaye NC, Dekker FW, Evans M, Caskey FJ, Torino C, Postorino M, et al. Renal function decline in older men and women with advanced chronic kidney disease-results from the EQUAL study. Nephrol Dial Transpl. 2021;36(9):1656–63.
Katz-Greenberg G, Shah S. Sex and gender differences in kidney transplantation. Semin Nephrol. 2022;42(2):219–29.
Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. J Clin Invest. 2019;129(5):1818–26.
Abramicheva PA, Plotnikov EY. Hormonal regulation of renal fibrosis. Life. 2022;12(5):737.
Ma HY, Chen S, Du Y. Estrogen and estrogen receptors in kidney diseases. Ren Fail. 2021;43(1):619–42.
Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33.
Ahmed SB, Culleton BF, Tonelli M, Klarenbach SW, Macrae JM, Zhang J, et al. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008;74(3):370–6.
Martínez-Klimova E, Aparicio-Trejo OE, Tapia E, Pedraza-Chaverri J. Unilateral ureteral obstruction as a model to investigate fibrosis-attenuating treatments. Biomolecules. 2019;9(4):141.
Vaughan ED, Marion D, Poppas DP, Felsen D. Pathophysiology of unilateral ureteral obstruction: studies from Charlottesville to New York. J Urol. 2004;172(6 Part 2):2563–9.
Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75(11):1145–52.
Dendooven A, Ishola DA Jr, Nguyen TQ, Van der Giezen DM, Kok RJ, Goldschmeding R, et al. Oxidative stress in obstructive nephropathy. Int J Exp Pathol. 2011;92(3):202–10.
Hosseinian S, Rad AK, Bideskan AE, Soukhtanloo M, Sadeghnia H, Shafei MN, et al. Thymoquinone ameliorates renal damage in unilateral ureteral obstruction in rats. Pharmacol Rep. 2017;69(4):648–57.
Wang H, Qian J, Zhao X, Xing C, Sun B. β-Aminoisobutyric acid ameliorates the renal fibrosis in mouse obstructed kidneys via inhibition of renal fibroblast activation and fibrosis. J Pharmacol Sci. 2017;133(4):203–13.
Klahr S, Harris K, Purkerson ML. Effects of obstruction on renal functions. Pediatr Nephrol. 1988;2:34–42.
Song J, He Y, Luo C, Feng B, Ran F, Xu H, et al. New progress in the pharmacology of protocatechuic acid: a compound ingested in daily foods and herbs frequently and heavily. Pharmacol Res. 2020;161: 105109.
Khan AK, Rashid R, Fatima N, Mahmood S, Mir S, Khan S, et al. Pharmacological activities of protocatechuic acid. Acta Pol Pharm. 2015;72(4):643–50.
Semaming Y, Pannengpetch P, Chattipakorn SC, Chattipakorn N. Pharmacological properties of protocatechuic Acid and its potential roles as complementary medicine. Evid Based Complement Alternat Med. 2015;2015: 593902.
Zhang S, Gai Z, Gui T, Chen J, Chen Q, Li Y. Antioxidant effects of protocatechuic acid and protocatechuic aldehyde: old wine in a new bottle. Evid Based Complement Alternat Med. 2021;2021:6139308.
Yang X, Sun X, Zhou F, Xiao S, Zhong L, Hu S, et al. Protocatechuic acid alleviates dextran-sulfate-sodium-induced ulcerative colitis in mice via the regulation of intestinal flora and ferroptosis. Molecules. 2023;28(9):3775.
Wan L, Li J, Chen WB, Wu GQ. Beneficial effects of protocatechuic acid on diabetic retinopathy in streptozocin-induced diabetic rats. Int J Ophthalmol. 2023;16(6):855–62.
Abdelrahman RS, El-Tanbouly GS. Protocatechuic acid protects against thioacetamide-induced chronic liver injury and encephalopathy in mice via modulating mTOR, p53 and the IL-6/ IL-17/ IL-23 immunoinflammatory pathway. Toxicol Appl Pharmacol. 2022;440: 115931.
Song H, Ren J. Protocatechuic acid attenuates angiotensin II-induced cardiac fibrosis in cardiac fibroblasts through inhibiting the NOX4/ROS/p38 signaling pathway. Phytother Res. 2019;33(9):2440–7.
Xiang Y, Huang R, Wang Y, Han S, Qin X, Li Z, et al. Protocatechuic acid ameliorates high fat diet-induced obesity and insulin resistance in mice. Mol Nutr Food Res. 2023;67(3): e2200244.
Kale S, Sarode LP, Kharat A, Ambulkar S, Prakash A, Sakharkar AJ, et al. Protocatechuic acid prevents early hour ischemic reperfusion brain damage by restoring imbalance of neuronal cell death and survival proteins. J Stroke Cerebrovasc Dis. 2021;30(2): 105507.
Salama AAA, Elgohary R, Fahmy MI. Protocatechuic acid ameliorates lipopolysaccharide-induced kidney damage in mice via downregulation of TLR-4-mediated IKBKB/NF-κB and MAPK/Erk signaling pathways. J Appl Toxicol. 2023;43(8):1119–29.
Yüksel M, Yıldar M, Başbuğ M, Çavdar F, Çıkman Ö, Akşit H, et al. Does protocatechuic acid, a natural antioxidant, reduce renal ischemia reperfusion injury in rats? Ulus Travma Acil Cerrahi Derg. 2017;23(1):1–6.
Gao L, Wu WF, Dong L, Ren GL, Li HD, Yang Q, et al. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Front Pharmacol. 2016;7:479.
Bai Y, Wang W, Yin P, Gao J, Na L, Sun Y, et al. Ruxolitinib alleviates renal interstitial fibrosis in UUO mice. Int J Biol Sci. 2020;16(2):194–203.
Makled MN, El-Kashef DH. Saroglitazar attenuates renal fibrosis induced by unilateral ureteral obstruction via inhibiting TGF-β/Smad signaling pathway. Life Sci. 2020;253: 117729.
Kassab RB, Theyab A, Al-Ghamdy AO, Algahtani M, Mufti AH, Alsharif KF, et al. Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ Sci Pollut Res Int. 2022;29(8):12208–21.
Mishra M, Tiwari S, Gomes AV. Protein purification and analysis: next generation Western blotting techniques. Expert Rev Proteomics. 2017;14(11):1037–53.
Jaffé M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Biol Chem. 1886;10(5):391–400.
Fernandes R, Garver H, Harkema JR, Galligan JJ, Fink GD, Xu H. Sex differences in renal inflammation and injury in high-fat diet-fed dahl salt-sensitive rats. Hypertension. 2018;72(5):e43–52.
Hosszu A, Fekete A, Szabo AJ. Sex differences in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2020;319(2):F149–54.
Ciarambino T, Crispino P, Giordano M. Gender and renal insufficiency: opportunities for their therapeutic management? Cells. 2022;11(23):3820.
Corcoran MP, Meydani M, Lichtenstein AH, Schaefer EJ, Dillard A, Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol. 2010;206(2):217–24.
Martínez de Toda I, González-Sánchez M, Díaz-Del Cerro E, Valera G, Carracedo J, Guerra-Pérez N. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mech Ageing Dev. 2023;211:111797.
Li L, Liu S, Tang H, Song S, Lu L, Zhang P, et al. Effects of protocatechuic acid on ameliorating lipid profiles and cardio-protection against coronary artery disease in high fat and fructose diet fed in rats. J Vet Med Sci. 2020;82(9):1387–94.
Baba I, Egi Y, Utsumi H, Kakimoto T, Suzuki K. Inhibitory effects of fasudil on renal interstitial fibrosis induced by unilateral ureteral obstruction. Mol Med Rep. 2015;12(6):8010–20.
Soji K, Doi S, Nakashima A, Sasaki K, Doi T, Masaki T. Deubiquitinase inhibitor PR-619 reduces Smad4 expression and suppresses renal fibrosis in mice with unilateral ureteral obstruction. PLoS ONE. 2018;13(8): e0202409.
Cavaglieri RC, Day RT, Feliers D, Abboud HE. Metformin prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. Mol Cell Endocrinol. 2015;412:116–22.
Garate-Carrillo A, Gonzalez J, Ceballos G, Ramirez-Sanchez I, Villarreal F. Sex related differences in the pathogenesis of organ fibrosis. Transl Res. 2020;222:41–55.
Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122(11 Suppl):S23–8.
Lee HW, Eghbali-Webb M. Estrogen enhances proliferative capacity of cardiac fibroblasts by estrogen receptor- and mitogen-activated protein kinase-dependent pathways. J Mol Cell Cardiol. 1998;30(7):1359–68.
Weinberg EO, Thienelt CD, Katz SE, Bartunek J, Tajima M, Rohrbach S, et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol. 1999;34(1):264–73.
Liu QH, Li DG, Huang X, Zong CH, Xu QF, Lu HM. Suppressive effects of 17beta-estradiol on hepatic fibrosis in CCl4-induced rat model. World J Gastroenterol. 2004;10(9):1315–20.
Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol (N Y). 2013;9(10):633–9.
Wang YJ, Wang SS, Bickel M, Guenzler V, Gerl M, Bissell DM. Two novel antifibrotics, HOE 077 and Safironil, modulate stellate cell activation in rat liver injury: differential effects in males and females. Am J Pathol. 1998;152(1):279–87.
Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, et al. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50(4):1173–9.
Cui B, Yang Z, Wang S, Guo M, Li Q, Zhang Q, et al. The protective role of protocatechuic acid against chemically induced liver fibrosis in vitro and in vivo. Pharmazie. 2021;76(5):232–8.
Li L, Ma H, Zhang Y, Jiang H, Xia B, Sberi HA, et al. Protocatechuic acid reverses myocardial infarction mediated by β-adrenergic agonist via regulation of Nrf2/HO-1 pathway, inflammatory, apoptotic, and fibrotic events. J Biochem Mol Toxicol. 2023;37(3): e23270.
Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302(11):F1351–61.
Wang H, Gao M, Li J, Sun J, Wu R, Han D, et al. MMP-9-positive neutrophils are essential for establishing profibrotic microenvironment in the obstructed kidney of UUO mice. Acta Physiol (Oxf). 2019;227(2): e13317.
Zhao H, Dong Y, Tian X, Tan TK, Liu Z, Zhao Y, et al. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J Nephrol. 2013;2(3):84–9.
Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA. 1996;93(14):7069–74.
Trentini A, Manfrinato MC, Castellazzi M, Bellini T. Sex-related differences of matrix metalloproteinases (MMPs): new perspectives for these biomarkers in cardiovascular and neurological diseases. J Pers Med. 2022;12(8):1196.
Lin HH, Chen JH, Chou FP, Wang CJ. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br J Pharmacol. 2011;162(1):237–54.
Singer E, Markó L, Paragas N, Barasch J, Dragun D, Müller DN, et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf). 2013;207(4):663–72.
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–43.
Latoch E, Konończuk K, Taranta-Janusz K, Muszyńska-Rosłan K, Szymczak E, Wasilewska A, et al. Urine NGAL and KIM-1: tubular injury markers in acute lymphoblastic leukemia survivors. Cancer Chemother Pharmacol. 2020;86(6):741–9.
Xiong Y, Chang Y, Hao J, Zhang C, Yang F, Wang Z, et al. Eplerenone attenuates fibrosis in the contralateral kidney of uuo rats by preventing macrophage-to-myofibroblast transition. Front Pharmacol. 2021;12:620433.
Jover E, Matilla L, Martín-Núñez E, Garaikoetxea M, Navarro A, Fernández-Celis A, et al. Sex-dependent expression of neutrophil gelatinase-associated lipocalin in aortic stenosis. Biol Sex Differ. 2022;13(1):71.
Viñas Jose L, Porter Christopher J, Douvris A, Spence M, Gutsol A, Zimpelmann Joseph A, et al. Sex diversity in proximal tubule and endothelial gene expression in mice with ischemic acute kidney injury. Clin Sci (Lond). 2020;134(14):1887–909.
Jin Y, Shao X, Sun B, Miao C, Li Z, Shi Y. Urinary kidney injury molecule-1 as an early diagnostic biomarker of obstructive acute kidney injury and development of a rapid detection method. Mol Med Rep. 2017;15(3):1229–35.
Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Niño MD, Sanz AB, Ramos AM, et al. Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol. 2014;46:765–76.
Aranda-Rivera AK, Cruz-Gregorio A, Aparicio-Trejo OE, Ortega-Lozano AJ, Pedraza-Chaverri J. Redox signaling pathways in unilateral ureteral obstruction (UUO)-induced renal fibrosis. Free Radic Biol Med. 2021;172:65–81.
Liu M, Zhu Y, Sun Y, Wen Z, Huang S, Ding G, et al. MnTBAP therapy attenuates the downregulation of sodium transporters in obstructive kidney disease. Oncotarget. 2018;9(1):394–403.
Barp J, Araújo AS, Fernandes TR, Rigatto KV, Llesuy S, Belló-Klein A, et al. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz J Med Biol Res. 2002;35(9):1075–81.
Matarrese P, Colasanti T, Ascione B, Margutti P, Franconi F, Alessandri C, et al. Gender disparity in susceptibility to oxidative stress and apoptosis induced by autoantibodies specific to RLIP76 in vascular cells. Antioxid Redox Signal. 2011;15(11):2825–36.
Bhatia K, Elmarakby AA, El-Remessy AB, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302(2):R274–82.
Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med. 2017;21(5):1024–32.
Chen J, Yang J, Ma L, Li J, Shahzad N, Kim CK. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci Rep. 2020;10(1):2611.
de Faria CM, Nazaré AC, Petrônio MS, Paracatu LC, Zeraik ML, Regasini LO, et al. Protocatechuic acid alkyl esters: hydrophobicity as a determinant factor for inhibition of NADPH oxidase. Curr Med Chem. 2012;19(28):4885–93.
Graton ME, Ferreira B, Troiano JA, Potje SR, Vale GT, Nakamune A, et al. Comparative study between apocynin and protocatechuic acid regarding antioxidant capacity and vascular effects. Front Physiol. 2022;13:1047916.
Abbas NAT, El Salem A, Awad MM. Empagliflozin, SGLT(2) inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: potential role of klotho expression. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(12):1347–60.
Wyczanska M, Lange-Sperandio B. DAMPs in unilateral ureteral obstruction. Front Immunol. 2020;11: 581300.
Boswell R, Yard B, Schrama E, Van Es L, Daha M, Van der Woude F. Interleukin 6 production by human proximal tubular epithelial cells in vitro: analysis of the effects of interleukin-1α (IL-1α) and other cytokines. Nephrol Dial Transplant. 1994;9(6):599–606.
Fan J-M, Huang X-R, Ng Y-Y, Nikolic-Paterson DJ, Mu W, Atkins RC, et al. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-β1-dependent mechanism in vitro. Am J Kidney Dis. 2001;37(4):820–31.
Jones LK, O’Sullivan KM, Semple T, Kuligowski MP, Fukami K, Ma FY, et al. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant. 2009;24(10):3024–32.
Gu L, Wang Y, Yang G, Tilyek A, Zhang C, Li S, et al. Ribes diacanthum Pall (RDP) ameliorates UUO-induced renal fibrosis via both canonical and non-canonical TGF-β signaling pathways in mice. J Ethnopharmacol. 2019;231:302–10.
Yang J, Chen J, Yan J, Zhang L, Chen G, He L, et al. Effect of interleukin 6 deficiency on renal interstitial fibrosis. PLoS ONE. 2012;7(12): e52415.
Chen W, Yuan H, Cao W, Wang T, Chen W, Yu H, et al. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics. 2019;9(14):3980–91.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26.
Munshi R, Johnson A, Siew ED, Ikizler TA, Ware LB, Wurfel MM, et al. MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol. 2011;22(1):165–75.
Chook CYB, Cheung YM, Ma KY, Leung FP, Zhu H, Niu QJ, et al. Physiological concentration of protocatechuic acid directly protects vascular endothelial function against inflammation in diabetes through Akt/eNOS pathway. Front Nutr. 2023;10:1060226.
Li P, Wang X, Wang H, Wu Y-N. High performance liquid chromatographic determination of phenolic acids in fruits and vegetables. Biomed Environ Sci. 1993;6(4):389–98.
Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26(1):99–114.
Zhang KJ, Wu Q, Jiang SM, Ding L, Liu CX, Xu M, et al. Pyroptosis: a new frontier in kidney diseases. Oxid Med Cell Longev. 2021;2021:6686617.
Xu Y, Ruan S, Wu X, Chen H, Zheng K, Fu B. Autophagy and apoptosis in tubular cells following unilateral ureteral obstruction are associated with mitochondrial oxidative stress. Int J Mol Med. 2013;31(3):628–36.
Cachat F, Lange-Sperandio B, Chang AY, Kiley SC, Thornhill BA, Forbes MS, et al. Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int. 2003;63(2):564–75.
Ortona E, Matarrese P, Malorni W. Taking into account the gender issue in cell death studies. Cell Death Dis. 2014;5(3):e1121.
Muley MM, Thakare VN, Patil RR, Bafna PA, Naik SR. Amelioration of cognitive, motor and endogenous defense functions with silymarin, piracetam and protocatechuic acid in the cerebral global ischemic rat model. Life Sci. 2013;93(1):51–7.
Adedara IA, Fasina OB, Ayeni MF, Ajayi OM, Farombi EO. Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem Toxicol. 2019;125:170–81.
Yamabe N, Park JY, Lee S, Cho E-J, Lee S, Kang KS, et al. Protective effects of protocatechuic acid against cisplatin-induced renal damage in rats. J Funct Foods. 2015;19:20–7.
Funding
This work was supported by intramural grants from Augusta University awarded to A.A.E. and B.B and grant from the Egyptian Cultural Affairs and Missions Sector (Cairo, Egypt) in cooperation with the Egyptian Cultural and Educational Bureau (Washington DC, USA) awarded to K.M.S.
Author information
Authors and Affiliations
Contributions
KMS contributed to mice surgical operations, biological sample collection, measurements, data analysis and manuscript drafting. ELS and SEN contributed to US Doppler measurements, biological sample collection and manuscript review. GMS, MEA, RRA and BB contributed to work conceptualization and manuscript review. AE contributed to experimental design, data analysis, manuscript drafting and review. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no competing interests that could have affected the conclusion of the current study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saad, K.M., Salles, É.L., Naeini, S.E. et al. Reno-protective effect of protocatechuic acid is independent of sex-related differences in murine model of UUO-induced kidney injury. Pharmacol. Rep 76, 98–111 (2024). https://doi.org/10.1007/s43440-023-00565-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00565-2