Abstract
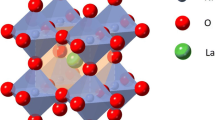
Searching for novel complex materials with enhanced lithium-ion battery performances is one of the most challenging efforts. Many kinds of transition metal oxides and polyanionic frameworks were developed with various structures, which can improve the energy density of lithium-ion batteries. In this work, we explored 4d and 4f transition metal La–Nb–O compounds as cathode materials for lithium-ion energy storage. Orthorhombic pyrochlore LaNb5O14, orthorhombic perovskite LaNb3O9, and monoclinic LaNbO4 compounds with different metal cation coordination polyhedra were synthesized using solid-state reaction. The orthorhombic pyrochlore LaNb5O14 compound showed the highest capacity among these La–Nb–O compounds owing to its quasi‐2D network for Li‐ion incorporation. According to the electronegativity theory and ionic size, La3+ cations can form LaO12 polyhedra and hexahedral LaO8 units in different La–Nb–O compounds, which can stabilize octahedral NbO6 and/or pentahedral NbO7 and their assembled structures, resulting in easy lithium-ion diffusion. This work may provide some structure clues for the design of electrode materials for fast lithium storage.








Similar content being viewed by others
Change history
21 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42864-021-00115-4
References
Harada JK, Charles N, Poeppelmeier KR, Rondinelli JM. Heteroanionic materials by design: progress toward targeted properties. Adv Mater. 2019;31(19):1805295.
Freire M, Kosova NV, Jordy C, Chateigner D, Lebedev OI, Maignan A, Pralong V. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat Mater. 2016;15:173.
Chen K, Song S, Liu F, Xue D. Structural design of graphene for use in electrochemical energy storage devices. Chem Soc Rev. 2015;44(17):6230.
Griffith KJ, Wiaderek KM, Cibin G, Marbella LE, Grey CP. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature. 2018;559:556.
Chen D, Wang JH, Chou TF, Zhao B, El-Sayed MA, Liu M. Unraveling the nature of anomalously fast energy storage in T-Nb2O5. J Am Chem Soc. 2017;139(20):7071.
Griffith KJ, Forse AC, Griffin JM, Grey CP. High-rate intercalation without nanostructuring in metastable Nb2O5 bronze phases. J Am Chem Soc. 2016;138(28):8888.
Odziomek M, Chaput F, Rutkowska A, Świerczek K, Olszewska D, Sitarz M, Lerouge F, Parola S. Hierarchically structured lithium titanate for ultrafast charging in long-life high capacity batteries. Nat Commun. 2017;8:15636.
Chen K, Xue D. Crystallization of transition metal oxides within 12 seconds. Cryst Eng Comm. 2017;19:1230.
Chen K, Xue D. Materials chemistry toward electrochemical energy storage. J Mater Chem A. 2016;4(20):7522.
Deng Q, Fu Y, Zhu C, Yu Y. Niobium-based oxides toward advanced electrochemical energy storage: recent advances and challenges. Small. 2019;15(32):1804884.
Miruszewski T, Winiarz P, Dzierzgowski K, Wiciak K, Zagórski K, Morawski A, Mielewczyk-Gryń A, Wachowski S, Strychalska-Nowak J, Sawczak M, Gazd M. Synthesis, microstructure and electrical properties of nanocrystalline calcium doped lanthanum orthoniobate. J Solid State Chem. 2019;270:601.
Wachowski S, Mielewczyk-Gryn A, Zagorski K, Li C, Jasinski P, Skinner SJ, Haugsrud R, Gazda M. Influence of Sb-substitution on ionic transport in lanthanum orthoniobates. J Mater Chem A. 2016;4:11696.
Brunckova H, Medvecky L, Kovalcikova A, Fides M, Mudra E, Durisin J, Sebek M, Kanuchova M, Skvarla J. Structural and mechanical properties of La1/3NbO3 and La1/3TaO3 thin films prepared by chemical solution deposition. J Rare Earths. 2017;35(11):1115.
Brunckova H, Medvecky L, Hvizdos P, Girman V. Effect of solvent on phase composition and particle morphology of lanthanum niobates prepared by polymeric complex sol–gel method. J Sol Gel Sci Technol. 2014;69(2):272.
Brunckova H, Medvecky L, Hvizdos P, Durisin J, Girman V. Structural and mechanical properties of sol–gel prepared pyrochlore lanthanum niobates. J Mater Sci. 2015;50(22):7197.
Li K, Shao J, Xue D. Site selectivity in doped polyanion cathode materials for Li-ion batteries. Funct Mater Lett. 2013;6(4):1350043.
Li K, Xue D. Estimation of electronegativity values of elements in different valence states. J Phys Chem A. 2006;110(39):11332.
Melot BC, Tarascon JM. Design and preparation of materials for advanced electrochemical storage. Acc Chem Res. 2013;46(5):1226.
Jehng JM, Wachs IE. Structural chemistry and Raman spectra of niobium oxides. Chem Mater. 1991;3(1):100.
Ishii K, Morita N, Nakayama K, Tsunekawa S, Fukuda T. Raman spectra of LaNbO4 in the ferroelastic phase and the relaxation after the state shift. Phys Status Solidi A. 1989;112(1):207.
Griffith KJ, Senyshyn A, Grey CP. Structural stability from crystallographic shear in TiO2–Nb2O5 phases: cation ordering and lithiation behavior of TiNb24O62. Inorg Chem. 2017;56(7):4002.
Cao Y, Duan N, Yan D, Chi B, Pu J, Jian L. Enhanced electrical conductivity of LaNbO4 by A-site substitution. Int J Hydrogen Energy. 2016;41(45):20633.
Deng T, Zhang W, Arcelus O, Kim JG, Carrasco J, Yoo SJ, Zheng W, Wang J, Tian H, Zhang H, Cui X, Rojo T. Atomic-level energy storage mechanism of cobalt hydroxide electrode for pseudocapacitors. Nat Commun. 2017;8:15194.
Chen K, Xue D. How to high-efficiently utilize electrode materials in supercapattery? Funct Mater Lett. 2019;12(1):1830005.
Lukatskaya MR, Kota S, Lin Z, Zhao MQ, Shpigel N, Levi MD, Halim J, Taberna PL, Barsoum MW, Simon P, Gogotsi Y. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat Energy. 2017;2:17105.
Liang X, Chen K, Xue D. A flexible and ultrahigh energy density capacitor via enhancing surface/interface of carbon cloth supported colloids. Adv Energy Mater. 2018;8(16):1703329.
Sun C, Xue D. Multisize and multiweight effects in materials science and engineering. Sci China Technol Sci. 2019;62(4):707.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21601176), CAS-VPST Silk Road Science Found 2018 (Grant No. GJHZ1854), the Youth Innovation Promotion Association, CAS (Grant No. 2018262), Jilin Province Youth Talent Lifting Project (Grant No. 181901), and the Youth Talent Development Program of the State Key Laboratory of Rare Earth Resource Utilization (Grant No. RERUY2017004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, K., Yin, S. & Xue, D. Active La–Nb–O compounds for fast lithium-ion energy storage. Tungsten 1, 287–296 (2019). https://doi.org/10.1007/s42864-019-00029-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42864-019-00029-2