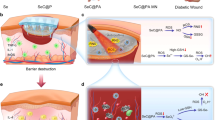
Abstract
Diabetic wounds have become a major clinical problem that cannot be ignored. Gases, such as hydrogen sulphide (H2S), have demonstrated value in inducing angiogenesis and accelerating wound healing, while their effective delivery is still challenging. Here, inspired by the continuous-independent hollow structure of bamboo, we propose novel gasotransmitter microfibres with septal H2S bubbles using microfluidic spinning for diabetic wound healing. Benefitting from the exact control of microfluidics, gasotransmitter microfibres with different bubble sizes and morphologies could be generated successfully and continuously. Under the dual effects of drugs in the shell and gas in the core, the wound healing process could be accelerated. Furthermore, the controllable release of drugs could be achieved by adding responsive materials into the microfiber shell, which would promote continuous effects of contents on demand. Based on in vitro and in vivo studies, we have proven that these gasotransmitter microfibres have a positive impact on inducing angiogenesis and promoting cell proliferation during wound healing. Thus, it is believed that the bamboo-inspired gasotransmitter microfibres will have important value in gasotransmitter research and clinical applications.
Graphical Abstract
The bamboo-inspired microfibres are presented through microfluidics with features of independent chambers for storing and controlled release of hydrogen sulphide (H2S) to the diabetic wound. Even if it is partially damaged, it will not affect the overall gas storage and utilization. Thus, it contributes to improvements in basic research and the transformation of gasotransmitters.







Similar content being viewed by others
Data availbility
The data that support the findings of this study are available from the authors, upon reasonable request.
References
Kharaziha M, Baidya A, Annabi N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater 2021;33(39):e2100176.
Matoori S, Veves A, Mooney D. Advanced bandages for diabetic wound healing. Sci Transl Med 2021;13(585):eabe4839.
Liu XT, Liu YM, Du JT, Li XR, Yu JY, Ding B. Breathable, stretchable and adhesive nanofibrous hydrogels as wound dressing materials. Eng Regen 2021;2:63–9.
da Silva LP, Reis RL, Correlo VM, Marques AP. A hydrogel-based strategies to advance therapies for chronic skin wounds. Annu Rev Biomed Eng 2019;21:145–69.
Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol 2019;21:18–24.
Hosseini M, Shafiee A. Engineering bioactive scaffolds for skin regeneration. Small 2021;17(41):e2101384.
Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev 2019;146:97–125.
Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Acc Chem Res 2021;54:1080–93.
Kargozar S, Baino F, Hamzehlou S, Hamblin MR, Mozafari M. Nanotechnology for angiogenesis: opportunities and challenges. Chem Soc Rev 2020;49(14):5008–57.
Lv B, Chen S, Tang C, Jin H, Du J, Huang Y. Hydrogen sulfide and vascular regulation—an update. J Adv Res 2021;27:85–97.
Tian D, Teng X, Jin S, Chen Y, Xue H, Xiao L, Wu Y. Endogenous hydrogen sulfide improves vascular remodeling through PPARδ/SOCS3 signaling. J Adv Res 2021;27:115–25.
Zhao H, Lu S, Chai J, Zhang Y, Ma X, Chen J, Guan Q, Wan M, Liu Y. Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J Diabetes Complicat 2017;31(9):1363–9.
Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, Chen AF. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 2014;63(6):1763–78.
Wu J, Chen A, Zhou Y, Zheng S, Yang Y, An Y, Xu K, He H, Kang J, Luckanagul JA, Xian M, Xiao J, Wang Q. Novel H2S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials 2019;222:119398.
Zhao X, Liu L, An T, Xian M, Luckanagul JA, Su Z, Lin Y, Wang Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater 2020;104:85–94.
Dakhchoune M, Villalobos LF, Semino R, Liu L, Rezaei M, Schouwink P, Avalos CE, Baade P, Wood V, Han Y, Ceriotti M, Agrawal KV. Gas-sieving zeolitic membranes fabricated by condensation of precursor nanosheets. Nat Mater 2021;20(3):362–9.
Kang J, Li Z, Organ CL, Park CM, Yang CT, Pacheco A, Wang D, Lefer DJ, Xian M. pH-Controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J Am Chem Soc 2016;138(20):6336–9.
Safonov M, You J, Lee J, Safonov VL, Berman D, Zhu D. Hydrogen generating patch improves skin cell viability, migration activity, and collagen expression. Eng Reg 2020;1:1–5.
Guo J, Yu Y, Wang H, Zhang H, Zhang X, Zhao Y. Conductive polymer hydrogel microfibers from multiflow microfluidics. Small 2019;15(15):e1805162.
Yu Y, Shang L, Guo J, Wang J, Zhao Y. Design of capillary microfluidics for spinning cell-laden microfibers. Nat Protoc 2018;13(11):2557–79.
Guo J, Yu Y, Zhang D, Zhang H, Zhao Y. Morphological hydrogel microfibers with MXene encapsulation for electronic skin. Research 2021;2021:7065907.
Meng LL, Zhang M, Deng HH, Xu BJ, Wang HQ, Wang YJ, Jiang L, Liu H. Direct-Writing large-area cross-aligned Ag nanowires network: toward high-performance transparent quantum dot light-emitting diodes. CCS Chem 2021;3(8):2194–202.
Ji XF, Li Z, Hu YB, Xie HL, Wu WJ, Song FY, Liu HX, Jiang MJ, Lam JWY, Tang BZ. Bioinspired hydrogels with muscle-like structure for AIEgen-guided selective self-healing. CCS Chem 2021;3(4):1146–56.
Li YJ, Ding YQ, Yang B, Cao TY, Xu JF, Dong YC, Chen Q, Xu LJ, Liu DS. Reinforcing DNA supramolecular hydrogel with polymeric multiple-unit linker. CCS Chem 2022. https://doi.org/10.31635/ccschem.022.202101523 .
Aleman J, Kilic T, Mille LS, Shin SR, Zhang YS. Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat Protoc 2021;16(5):2564–93.
Zhang X, Wang Z, Zhang YS, Yan S, Hou C, Gong Y, Qiu J, Chen M, Li Q. Studying endothelial cell shedding and orientation using adaptive perfusion-culture in a microfluidic vascular chip. Biotechnol Bioeng 2021;118(2):963–78.
Yu Y, Guo J, Sun L, Zhang X, Zhao Y. Microfluidic generation of microsprings with ionic liquid encapsulation for flexible electronics. Research 2019;2019:6906275.
Yu Y, Fu F, Shang L, Cheng Y, Gu Z, Zhao Y. Bioinspired helical microfibers from microfluidics. Adv Mater 2017. https://doi.org/10.1002/adma.201605765 .
Zhao C, Chen G, Wang H, Zhao Y, Chai R. Bio-inspired intestinal scavenger from microfluidic electrospray for detoxifying lipopolysaccharide. Bioact Mater 2020;6(6):1653–62.
Liang XC, Kumar V, Ahmadi F, Zhu YY. Manipulation of droplets and bubbles for thermal applications. Droplet 2022. https://doi.org/10.1002/dro2.21 .
Wang Z, Wang Y, Yan J, Zhang K, Lin F, Xiang L, Deng L, Guan Z, Cui W, Zhang H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev 2021;174:504–34.
Wang Y, Lu L, Zheng G, Zhang X. Microenvironment-Controlled micropatterned microfluidic model (MMMM) for biomimetic in situ studies. ACS Nano 2020;14(8):9861–72.
Wang P, Bian R, Meng Q, Liu H, Jiang L. Bioinspired dynamic wetting on multiple fibers. Adv Mater 2017;29(45):1703042.
Wang P, Zhou J, Xu B, Lu C, Meng Q, Liu H. Bioinspired anti-Plateau-Rayleigh-instability on dual parallel fibers. Adv Mater 2020;32(45):e2003453.
Liu W, Zhao L, Wang C, Zhou J. Conductive nanomaterials for cardiac tissues engineering. Eng Regen 2020;1:88–94.
Kim YI, Kim MW, An S, Yarin AL, Yoon SS. Reusable filters augmented with heating microfibers for antibacterial and antiviral sterilization. ACS Appl Mater Interfaces 2021;13(1):857–67.
Tao W, Kong N, Ji X, Zhang Y, Sharma A, Ouyang J, Qi B, Wang J, Xie N, Kang C, Zhang H, Farokhzad OC, Kim JS. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem Soc Rev 2019;48(11):2891–912.
Sun L, Fan L, Bian F, Chen G, Wang Y, Zhao Y. MXene-Integrated microneedle patches with innate molecule encapsulation for wound healing. Research 2021;2021:9838490.
Romo-Uribe A, Albanil L. POSS-Induced dynamic cross-links produced self-healing and shape memory physical hydrogels when copolymerized with N-Isopropyl acrylamide. ACS Appl Mater Interfaces 2019;11(27):24447–58.
Breger JC, Yoon C, Xiao R, Kwag HR, Wang MO, Fisher JP, Nguyen TD, Gracias DH. Self-folding thermo-magnetically responsive soft microgrippers. ACS Appl Mater Interfaces 2015;7(5):3398–405.
Wang FW, Hsu CW, Hsieh CC. Numerical design and experimental realization of a PNIPAM-based micro thermosensor. ACS Appl Mater Interfaces 2019;11(8):8591–600.
Yang Y, Zhang Q, Xu T, Zhang H, Zhang M, Lu L, Hao Y, Fuh JH, Zhao X. Photocrosslinkable nanocomposite ink for printing strong, biodegradable and bioactive bone graft. Biomaterials 2020;263:120378.
Zhang H, Chen G, Yu Y, Guo J, Tan Q, Zhao Y. Microfluidic printing of slippery textiles for medical drainage around wounds. Adv Sci 2020;7(16):2000789.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2020YFA0908200), the National Science Foundation of China (52073060 and 61927805), National Major New Drug Innovation Science and Technology Major Project (2019ZX09301132), Guangdong Basic and Applied Basic Research Foundation (2021B1515120054, 2019A1515110925), and the Shenzhen Fundamental Research Program (JCYJ20190813152616459 and JCYJ20210324133214038).
Author information
Authors and Affiliations
Contributions
YJZ conceived the study and participated in its design. CZ conducted the experiments, wrote this manuscript and finished all figures in this article. JHG, HZ, YJZ, CHY and LPZ contributed to scientific discussion of the article. YJZ and LPZ helped finish the animal experiments. MN completed the cell experiment in this research.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, C., Guo, J., Zhang, H. et al. Bamboo-Inspired Gasotransmitter Microfibres for Wound Healing. Adv. Fiber Mater. 5, 388–399 (2023). https://doi.org/10.1007/s42765-022-00235-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00235-7