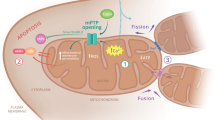
Abstract
It has been well-acknowledged that N-methyl-D-aspartate receptors (NMDARs) in central nervous system play an important role in both physiological functions of neurons and the pathophysiological progression of various neurodegenerative diseases. Besides, the receptors have also been found to present extensively in peripheral organs, such as lungs, kidneys, heart, and pancreas. Under various pathological conditions, peripheral NMDARs are upregulated and excessively activated, initiating calcium influx and intracellular calcium overloading. Subsequently, mitochondrial dysfunction, oxidative stress, and pro-inflammatory signaling pathway activation ultimately aggravate tissue damage and organ dysfunction. In addition, excessive activation of NMDARs also directly initiates mitochondrial apoptosis in many organs. Here, we discuss pathophysiological roles of NMDARs in cardiovascular system, lungs, kidneys, and pancreas.


Similar content being viewed by others
References
Bartlett TE, Wang YT. The intersections of NMDAR-dependent synaptic plasticity and cell survival. Neuropharmacology. 2013;74:59–68. https://doi.org/10.1016/j.neuropharm.2013.01.012.
Lin CH, Huang YJ, Lin CJ, Lane HY, Tsai GE. NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer's disease. Curr Pharm Des. 2014;20(32):5169–79. https://doi.org/10.2174/1381612819666140110115603.
Aida T, Ito Y, Takahashi YK, Tanaka K. Overstimulation of NMDA receptors impairs early brain development in vivo. PLoS One. 2012;7(5):e36853. https://doi.org/10.1371/journal.pone.0036853.
Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, et al. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer's disease, vascular dementia and Parkinson's disease. Curr Alzheimer Res. 2012;9(6):746–58. https://doi.org/10.2174/156720512801322564.
Gonzalez J, Jurado-Coronel JC, Avila MF, Sabogal A, Capani F, Barreto GE. NMDARs in neurological diseases: a potential therapeutic target. Int J Neurosci. 2015;125(5):315–27. https://doi.org/10.3109/00207454.2014.940941.
Marquard J, Otter S, Welters A, Stirban A, Fischer A, Eglinger J, et al. Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat Med. 2015;21(4):363–72. https://doi.org/10.1038/nm.3822.
Santiago AR, Gaspar JM, Baptista FI, Cristovao AJ, Santos PF, Kamphuis W, et al. Diabetes changes the levels of ionotropic glutamate receptors in the rat retina. Mol Vis. 2009;15:1620–30.
Dryer SE. Glutamate receptors in the kidney. Nephrol Dial Transplant. 2015;30(10):1630–8. https://doi.org/10.1093/ndt/gfv028.
Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411–7. https://doi.org/10.1080/01926230701230361.
Hogan-Cann AD, Anderson CM. Physiological roles of non-neuronal NMDA receptors. Trends Pharmacol Sci. 2016;37(9):750–67. https://doi.org/10.1016/j.tips.2016.05.012.
Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–203.
Misra C, Restituito S, Ferreira J, Rameau GA, Fu J, Ziff EB. Regulation of synaptic structure and function by palmitoylated AMPA receptor binding protein. Mol Cell Neurosci. 2010;43(4):341–52. https://doi.org/10.1016/j.mcn.2010.01.001.
Millan MJ. N-methyl-D-aspartate receptor-coupled glycineB receptors in the pathogenesis and treatment of schizophrenia: a critical review. Curr Drug Targets CNS Neurol Disord. 2002;1(2):191–213. https://doi.org/10.2174/1568007024606258.
Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. https://doi.org/10.1038/nrn3504.
Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013;19(1):62–75. https://doi.org/10.1177/1073858411435129.
Wyllie DJ, Livesey MR, Hardingham GE. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology. 2013;74:4–17. https://doi.org/10.1016/j.neuropharm.2013.01.016.
Cavara NA, Hollmann M. Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol. 2008;38(1):16–26. https://doi.org/10.1007/s12035-008-8029-9.
Pachernegg S, Strutz-Seebohm N, Hollmann M. GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci. 2012;35(4):240–9. https://doi.org/10.1016/j.tins.2011.11.010.
Madry C, Betz H, Geiger JR, Laube B. Potentiation of glycine-gated NR1/NR3A NMDA receptors relieves Ca-dependent outward rectification. Front Mol Neurosci. 2010;3:6. https://doi.org/10.3389/fnmol.2010.00006.
Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ, et al. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol. 2018;150(8):1081–105. https://doi.org/10.1085/jgp.201812032.
Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344(6187):992–7. https://doi.org/10.1126/science.1251915.
Lee CH, Lu W, Michel JC, Goehring A, Du J, Song X, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511(7508):191–7. https://doi.org/10.1038/nature13548.
Zhu S, Paoletti P. Allosteric modulators of NMDA receptors: multiple sites and mechanisms. Curr Opin Pharmacol. 2015;20:14–23. https://doi.org/10.1016/j.coph.2014.10.009.
Shang LH, Luo ZQ, Deng XD, Wang MJ, Huang FR, Feng DD, et al. Expression of N-methyl-D-aspartate receptor and its effect on nitric oxide production of rat alveolar macrophages. Nitric Oxide. 2010;23(4):327–31. https://doi.org/10.1016/j.niox.2010.09.004.
Shen L, Li L, She H, Yue S, Li C, Luo Z. Inhibition of pulmonary surfactants synthesis during N-methyl-D-aspartate-induced lung injury. Basic Clin Pharmacol Toxicol. 2010;107(3):751–7. https://doi.org/10.1111/j.1742-7843.2010.00572.x.
Dickman KG, Youssef JG, Mathew SM, Said SI. Ionotropic glutamate receptors in lungs and airways: molecular basis for glutamate toxicity. Am J Respir Cell Mol Biol. 2004;30(2):139–44. https://doi.org/10.1165/rcmb.2003-0177OC.
Li X, Li C, Tang Y, Huang Y, Cheng Q, Huang X, et al. NMDA receptor activation inhibits the antifibrotic effect of BM-MSCs on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315(3):L404–L21. https://doi.org/10.1152/ajplung.00002.2018.
Said SI. Glutamate receptors and asthmatic airway disease. Trends Pharmacol Sci. 1999;20(4):132–4. https://doi.org/10.1016/s0165-6147(98)01275-9.
Polak MJ, Xiao S, Ashton CA, Baylis C. Nmda alters the development of hypoxic pulmonary vasoconstriction and nitric oxide synthetase activity in the isolated perfused rat lung. Exp Lung Res. 2002;28(3):251–63. https://doi.org/10.1080/019021402753570536.
Wang M, Luo Z, Yue Y, Wang Y, Wu S, Cao C, et al. The excitotoxity of NMDA receptor NR2D subtype mediates human fetal lung fibroblasts proliferation and collagen production. Toxicol in Vitro. 2018;46:47–57. https://doi.org/10.1016/j.tiv.2017.10.008.
Liao Z, Zhou X, Luo Z, Huo H, Wang M, Yu X, et al. N-methyl-D-aspartate receptor excessive activation inhibited fetal rat lung development in vivo and in vitro. Biomed Res Int. 2016;2016:5843981–11. https://doi.org/10.1155/2016/5843981.
Robertson BS, Satterfield BE, Said SI, Dey RD. N-methyl-D-aspartate receptors are expressed by intrinsic neurons of rat larynx and esophagus. Neurosci Lett. 1998;244(2):77–80. https://doi.org/10.1016/s0304-3940(98)00130-x.
Leung JC, Travis BR, Verlander JW, Sandhu SK, Yang SG, Zea AH, et al. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am J Physiol Regul Integr Comp Physiol. 2002;283(4):R964–71. https://doi.org/10.1152/ajpregu.00629.2001.
Nesterov SV, Skorobogatova YA, Panteleeva AA, Pavlik LL, Mikheeva IB, Yaguzhinsky LS, et al. NMDA and GABA receptor presence in rat heart mitochondria. Chem Biol Interact. 2018;291:40–6. https://doi.org/10.1016/j.cbi.2018.06.004.
Makhro A, Tian Q, Kaestner L, Kosenkov D, Faggian G, Gassmann M, et al. Cardiac N-methyl D-aspartate receptors as a pharmacological target. J Cardiovasc Pharmacol. 2016;68(5):356–73. https://doi.org/10.1097/FJC.0000000000000424.
Rastaldi MP, Armelloni S, Berra S, Calvaresi N, Corbelli A, Giardino LA, et al. Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J. 2006;20(7):976–8. https://doi.org/10.1096/fj.05-4962fje.
Ma MC, Huang HS, Chen YS, Lee SH. Mechanosensitive N-methyl-D-aspartate receptors contribute to sensory activation in the rat renal pelvis. Hypertension. 2008;52(5):938–44. https://doi.org/10.1161/HYPERTENSIONAHA.108.114116.
Yang CC, Chien CT, Wu MH, Ma MC, Chen CF. NMDA receptor blocker ameliorates ischemia-reperfusion-induced renal dysfunction in rat kidneys. Am J Physiol Renal Physiol. 2008;294(6):F1433–40. https://doi.org/10.1152/ajprenal.00481.2007.
Zhang C, Yi F, Xia M, Boini KM, Zhu Q, Laperle LA, et al. NMDA receptor-mediated activation of NADPH oxidase and glomerulosclerosis in hyperhomocysteinemic rats. Antioxid Redox Signal. 2010;13(7):975–86. https://doi.org/10.1089/ars.2010.3091.
Giardino L, Armelloni S, Corbelli A, Mattinzoli D, Zennaro C, Guerrot D, et al. Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J Am Soc Nephrol. 2009;20(9):1929–40. https://doi.org/10.1681/ASN.2008121286.
Deng A, Thomson SC. Renal NMDA receptors independently stimulate proximal reabsorption and glomerular filtration. Am J Physiol Renal Physiol. 2009;296(5):F976–82. https://doi.org/10.1152/ajprenal.90391.2008.
Roshanravan H, Kim EY, Dryer SE. NMDA receptors as potential therapeutic targets in diabetic nephropathy: increased renal NMDA receptor subunit expression in Akita mice and reduced nephropathy following sustained treatment with memantine or MK-801. Diabetes. 2016;65(10):3139–50. https://doi.org/10.2337/db16-0209.
Kim EY, Anderson M, Dryer SE. Sustained activation of N-methyl-D-aspartate receptors in podoctyes leads to oxidative stress, mobilization of transient receptor potential canonical 6 channels, nuclear factor of activated T cells activation, and apoptotic cell death. Mol Pharmacol. 2012;82(4):728–37. https://doi.org/10.1124/mol.112.079376.
Bozic M, de Rooij J, Parisi E, Ortega MR, Fernandez E, Valdivielso JM. Glutamatergic signaling maintains the epithelial phenotype of proximal tubular cells. J Am Soc Nephrol. 2011;22(6):1099–111. https://doi.org/10.1681/ASN.2010070701.
Sproul A, Steele SL, Thai TL, Yu S, Klein JD, Sands JM, et al. N-methyl-D-aspartate receptor subunit NR3a expression and function in principal cells of the collecting duct. Am J Physiol Renal Physiol. 2011;301(1):F44–54. https://doi.org/10.1152/ajprenal.00666.2010.
Inagaki N, Kuromi H, Gonoi T, Okamoto Y, Ishida H, Seino Y, et al. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995;9(8):686–91.
Gonoi T, Mizuno N, Inagaki N, Kuromi H, Seino Y, Miyazaki J, et al. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem. 1994;269(25):16989–92.
Molnar E, Varadi A, McIlhinney RA, Ashcroft SJ. Identification of functional ionotropic glutamate receptor proteins in pancreatic beta-cells and in islets of Langerhans. FEBS Lett. 1995;371(3):253–7. https://doi.org/10.1016/0014-5793(95)00890-l.
Wu Y, Fortin DA, Cochrane VA, Chen PC, Shyng SL. NMDA receptors mediate leptin signaling and regulate potassium channel trafficking in pancreatic beta-cells. J Biol Chem. 2017;292(37):15512–24. https://doi.org/10.1074/jbc.M117.802249.
Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325(6104):529–31. https://doi.org/10.1038/325529a0.
Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241(4867):835–7. https://doi.org/10.1126/science.2841759.
Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415(6873):793–8. https://doi.org/10.1038/nature715.
Chen PE, Geballe MT, Katz E, Erreger K, Livesey MR, O'Toole KK, et al. Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J Physiol. 2008;586(1):227–45. https://doi.org/10.1113/jphysiol.2007.143172.
Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, et al. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Mol Pharmacol. 2007;72(4):907–20. https://doi.org/10.1124/mol.107.037333.
Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+. Neuron. 1995;15(5):1109–20. https://doi.org/10.1016/0896-6273(95)90099-3.
Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, et al. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun. 2010;1(1):90. https://doi.org/10.1038/ncomms1085.
Koch A, Bonus M, Gohlke H, Klocker N. Isoform-specific inhibition of N-methyl-D-aspartate receptors by bile salts. Sci Rep. 2019;9(1):10068. https://doi.org/10.1038/s41598-019-46496-y.
Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48(3):354–9. https://doi.org/10.1016/j.neuropharm.2004.11.004.
Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21(1):165–204. https://doi.org/10.1146/annurev.pa.21.040181.001121.
Costa BM, Feng B, Tsintsadze TS, Morley RM, Irvine MW, Tsintsadze V, et al. N-methyl-D-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J Pharmacol Exp Ther. 2009;331(2):618–26. https://doi.org/10.1124/jpet.109.156752.
Lodge D, Johnson KM. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990;11(2):81–6. https://doi.org/10.1016/0165-6147(90)90323-z.
Anis N, Sherby S, Goodnow R Jr, Niwa M, Konno K, Kallimopoulos T, et al. Structure-activity relationships of philanthotoxin analogs and polyamines on N-methyl-D-aspartate and nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1990;254(3):764–73.
Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986;83(18):7104–8. https://doi.org/10.1073/pnas.83.18.7104.
Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7(8):742–55. https://doi.org/10.1016/S1474-4422(08)70165-0.
Legendre P, Westbrook GL. Ifenprodil blocks N-methyl-D-aspartate receptors by a two-component mechanism. Mol Pharmacol. 1991;40(2):289–98.
Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475(7355):249–53. https://doi.org/10.1038/nature10180.
Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, et al. Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B-containing receptors. J Pharmacol Exp Ther. 2010;335(3):636–44. https://doi.org/10.1124/jpet.110.172544.
Edman S, McKay S, Macdonald LJ, Samadi M, Livesey MR, Hardingham GE, et al. TCN 201 selectively blocks GluN2A-containing NMDARs in a GluN1 co-agonist dependent but non-competitive manner. Neuropharmacology. 2012;63(3):441–9. https://doi.org/10.1016/j.neuropharm.2012.04.027.
Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, et al. Quinazolin-4-one derivatives: a novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J Med Chem. 2010;53(15):5476–90. https://doi.org/10.1021/jm100027p.
Hansen KB, Traynelis SF. Structural and mechanistic determinants of a novel site for noncompetitive inhibition of GluN2D-containing NMDA receptors. J Neurosci. 2011;31(10):3650–61. https://doi.org/10.1523/JNEUROSCI.5565-10.2011.
Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, et al. Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol Pharmacol. 2011;80(5):782–95. https://doi.org/10.1124/mol.111.073239.
Irvine MW, Costa BM, Volianskis A, Fang G, Ceolin L, Collingridge GL, et al. Coumarin-3-carboxylic acid derivatives as potentiators and inhibitors of recombinant and native N-methyl-D-aspartate receptors. Neurochem Int. 2012;61(4):593–600. https://doi.org/10.1016/j.neuint.2011.12.020.
Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, et al. A novel family of negative and positive allosteric modulators of NMDA receptors. J Pharmacol Exp Ther. 2010;335(3):614–21. https://doi.org/10.1124/jpet.110.174144.
Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Laube B, et al. Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacology. 2012;62(4):1730–6. https://doi.org/10.1016/j.neuropharm.2011.11.019.
Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci. 1998;18(16):6163–75.
Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol. 1997;51(6):1015–23. https://doi.org/10.1124/mol.51.6.1015.
Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17(15):5711–25.
Petrovic M, Sedlacek M, Horak M, Chodounska H, Vyklicky L Jr. 20-oxo-5beta-pregnan-3alpha-yl sulfate is a use-dependent NMDA receptor inhibitor. J Neurosci. 2005;25(37):8439–50. https://doi.org/10.1523/JNEUROSCI.1407-05.2005.
Borovska J, Vyklicky V, Stastna E, Kapras V, Slavikova B, Horak M, et al. Access of inhibitory neurosteroids to the NMDA receptor. Br J Pharmacol. 2012;166(3):1069–83. https://doi.org/10.1111/j.1476-5381.2011.01816.x.
Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52(6):1113–23. https://doi.org/10.1124/mol.52.6.1113.
Allen TC, Kurdowska A. Interleukin 8 and acute lung injury. Arch Pathol Lab Med. 2014;138(2):266–9. https://doi.org/10.5858/arpa.2013-0182-RA.
Yang HH, Hou CC, Lin MT, Chang CP. Attenuating heat-induced acute lung inflammation and injury by dextromethorphan in rats. Am J Respir Cell Mol Biol. 2012;46(3):407–13. https://doi.org/10.1165/rcmb.2011-0226OC.
Zhe Z, Hongyuan B, Wenjuan Q, Peng W, Xiaowei L, Yan G. Blockade of glutamate receptor ameliorates lipopolysaccharide-induced sepsis through regulation of neuropeptides. Biosci Rep. 2018;38(3). https://doi.org/10.1042/BSR20171629.
Wang M, Luo Z, Liu S, Li L, Deng X, Huang F, et al. Glutamate mediates hyperoxia-induced newborn rat lung injury through N-methyl-D-aspartate receptors. Am J Respir Cell Mol Biol. 2009;40(3):260–7. https://doi.org/10.1165/rcmb.2008-0135OC.
Li Y, Liu Y, Peng X, Liu W, Zhao F, Feng D, et al. NMDA receptor antagonist attenuates bleomycin-induced acute lung injury. PLoS One. 2015;10(5):e0125873. https://doi.org/10.1371/journal.pone.0125873.
da Cunha AA, Pauli V, Saciura VC, Pires MG, Constantino LC, de Souza B, et al. N-methyl-D-aspartate glutamate receptor blockade attenuates lung injury associated with experimental sepsis. Chest. 2010;137(2):297–302. https://doi.org/10.1378/chest.09-1570.
Hamidi SA, Dickman KG, Berisha H, Said SI. 17beta-estradiol protects the lung against acute injury: possible mediation by vasoactive intestinal polypeptide. Endocrinology. 2011;152(12):4729–37. https://doi.org/10.1210/en.2011-1631.
Said SI, Berisha HI, Pakbaz H. Excitotoxicity in the lung: N-methyl-D-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly (ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1996;93(10):4688–92. https://doi.org/10.1073/pnas.93.10.4688.
Tang F, Yue S, Luo Z, Feng D, Wang M, Qian C, et al. Role of N-methyl-D-aspartate receptor in hyperoxia-induced lung injury. Pediatr Pulmonol. 2005;40(5):437–44. https://doi.org/10.1002/ppul.20299.
Li JT, Wang WQ, Wang L, Liu NN, Zhao YL, Zhu XS, et al. Subanesthetic isoflurane relieves zymosan-induced neutrophil inflammatory response by targeting NMDA glutamate receptor and Toll-like receptor 2 signaling. Oncotarget. 2016;7(22):31772–89. https://doi.org/10.18632/oncotarget.9091.
da Cunha AA, Nunes FB, Lunardelli A, Pauli V, Amaral RH, de Oliveira LM, et al. Treatment with N-methyl-D-aspartate receptor antagonist (MK-801) protects against oxidative stress in lipopolysaccharide-induced acute lung injury in the rat. Int Immunopharmacol. 2011;11(6):706–11. https://doi.org/10.1016/j.intimp.2011.01.016.
Hamasato EK, Ligeiro de Oliveira AP, Lino-dos-Santos-Franco A, Ribeiro A, Ferraz de Paula V, Peron JP, et al. Effects of MK-801 and amphetamine treatments on allergic lung inflammatory response in mice. Int Immunopharmacol. 2013;16(4):436–43. https://doi.org/10.1016/j.intimp.2013.04.019.
Ben-Abraham R, Guttman M, Flaishon R, Marouani N, Niv D, Weinbroum AA. Mesenteric artery clamping/unclamping-induced acute lung injury is attenuated by N-methyl-D-aspartate antagonist dextromethorphan. Lung. 2006;184(6):309–17. https://doi.org/10.1007/s00408-006-0029-9.
Cheng Q, Fang L, Feng D, Tang S, Yue S, Huang Y, et al. Memantine ameliorates pulmonary inflammation in a mice model of COPD induced by cigarette smoke combined with LPS. Biomed Pharmacother. 2019;109:2005–13. https://doi.org/10.1016/j.biopha.2018.11.002.
Wang Y, Yue S, Luo Z, Cao C, Yu X, Liao Z, et al. N-methyl-D-aspartate receptor activation mediates lung fibroblast proliferation and differentiation in hyperoxia-induced chronic lung disease in newborn rats. Respir Res. 2016;17(1):136. https://doi.org/10.1186/s12931-016-0453-1.
Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510(Pt 1 1):1–18. https://doi.org/10.1111/j.1469-7793.1998.001bz.x.
McGee MA, Abdel-Rahman AA. Enhanced vascular neuronal nitric-oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-methyl-D-aspartate receptor activation in conscious rats. J Pharmacol Exp Ther. 2012;342(2):461–71. https://doi.org/10.1124/jpet.112.194464.
Taguchi K, Tamba M, Bannai S, Sato H. Induction of cystine/glutamate transporter in bacterial lipopolysaccharide induced endotoxemia in mice. J Inflamm (Lond). 2007;4(1):20. https://doi.org/10.1186/1476-9255-4-20.
Said SI, Pakbaz H, Berisha HI, Raza S. NMDA receptor activation: critical role in oxidant tissue injury. Free Radic Biol Med. 2000;28(8):1300–2. https://doi.org/10.1016/s0891-5849(00)00289-6.
Ma L, Liu W, Feng D, Han J, Li Y, Cheng Q, et al. Protective effect of NMDA receptor antagonist memantine on acute lung injury in mice. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39(1):12–6. https://doi.org/10.11817/j.issn.1672-7347.2014.01.003.
Shen L, Han JZ, Li C, Yue SJ, Liu Y, Qin XQ, et al. Protective effect of ginsenoside Rg1 on glutamate-induced lung injury. Acta Pharmacol Sin. 2007;28(3):392–7. https://doi.org/10.1111/j.1745-7254.2007.00511.x.
Jiang J, Jian Q, Jing M, Zhang Z, Zhang G, Shan L, et al. The novel N-methyl-d-aspartate receptor antagonist MN-08 ameliorates lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2019;66:109–18. https://doi.org/10.1016/j.intimp.2018.11.010.
Shi S, Liu T, Wang D, Zhang Y, Liang J, Yang B, et al. Activation of N-methyl-d-aspartate receptors reduces heart rate variability and facilitates atrial fibrillation in rats. Europace. 2017;19(7):1237–43. https://doi.org/10.1093/europace/euw086.
Shi S, Liu T, Li Y, Qin M, Tang Y, Shen JY, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367–77. https://doi.org/10.1111/pace.12430.
McGee MA, Abdel-Rahman AA. Ethanol attenuates peripheral NMDAR-mediated vascular oxidative stress and pressor response. Alcohol. 2015;49(5):499–506. https://doi.org/10.1016/j.alcohol.2015.03.004.
Lu J, Gao X, Gu J, Zhou L, Guo S, Hao W, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448–55. https://doi.org/10.1007/s12031-012-9720-x.
Liu Y, Zhou L, Xu HF, Yan L, Ding F, Hao W, et al. A preliminary experimental study on the cardiac toxicity of glutamate and the role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor in rats. Chin Med J. 2013;126(7):1323–32.
D'Amico M, Di Filippo C, Rossi F, Rossi F. Arrhythmias induced by myocardial ischaemia-reperfusion are sensitive to ionotropic excitatory amino acid receptor antagonists. Eur J Pharmacol. 1999;366(2–3):167–74. https://doi.org/10.1016/s0014-2999(98)00914-5.
Sun X, Zhong J, Wang D, Xu J, Su H, An C, et al. Increasing glutamate promotes ischemia-reperfusion-induced ventricular arrhythmias in rats in vivo. Pharmacology. 2014;93(1–2):4–9. https://doi.org/10.1159/000356311.
Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79(3):917–1017. https://doi.org/10.1152/physrev.1999.79.3.917.
Bennett AJ, DePetrillo PB. Differential effects of MK801 and lorazepam on heart rate variability in adolescent rhesus monkeys (macaca mulatta). J Cardiovasc Pharmacol. 2005;45(5):383–8. https://doi.org/10.1097/01.fjc.0000156820.12339.db.
Rosenberger D, Moshal KS, Kartha GK, Tyagi N, Sen U, Lominadze D, et al. Arrhythmia and neuronal/endothelial myocyte uncoupling in hyperhomocysteinemia. Arch Physiol Biochem. 2006;112(4–5):219–27. https://doi.org/10.1080/13813450601093443.
Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25(6):475–84. https://doi.org/10.1016/j.tcm.2014.12.015.
Gemel J, Levy AE, Simon AR, Bennett KB, Ai X, Akhter S, et al. Connexin40 abnormalities and atrial fibrillation in the human heart. J Mol Cell Cardiol. 2014;76:159–68. https://doi.org/10.1016/j.yjmcc.2014.08.021.
Meneghini A, Ferreira C, Abreu LC, Ferreira M, Ferreira Filho C, Valenti VE, et al. Cold stress effects on cardiomyocytes nuclear size in rats: light microscopic evaluation. Rev Bras Cir Cardiovasc. 2008;23(4):530–3. https://doi.org/10.1590/s0102-76382008000400013.
Meneghini A, Ferreira C, Abreu LC, Valenti VE, Ferreira M, Filho CF, et al. Memantine prevents cardiomyocytes nuclear size reduction in the left ventricle of rats exposed to cold stress. Clinics (Sao Paulo). 2009;64(9):921–6. https://doi.org/10.1590/S1807-59322009000900014.
Abbaszadeh S, Javidmehr A, Askari B, Janssen PML, Soraya H. Memantine, an NMDA receptor antagonist, attenuates cardiac remodeling, lipid peroxidation and neutrophil recruitment in heart failure: a cardioprotective agent? Biomed Pharmacother. 2018;108:1237–43. https://doi.org/10.1016/j.biopha.2018.09.153.
Matsuoka N, Kodama H, Arakawa H, Yamaguchi I. N-methyl-D-aspartate receptor blockade by dizocilpine prevents stress-induced sudden death in cardiomyopathic hamsters. Brain Res. 2002;944(1–2):200–4. https://doi.org/10.1016/s0006-8993(02)02885-8.
Tejero-Taldo MI, Chmielinska JJ, Gonzalez G, Mak IT, Weglicki WB. N-methyl-D-aspartate receptor blockade inhibits cardiac inflammation in the Mg2+−deficient rat. J Pharmacol Exp Ther. 2004;311(1):8–13. https://doi.org/10.1124/jpet.104.070003.
Liu ZY, Hu S, Zhong QW, Tian CN, Ma HM, Yu JJ. N-methyl-D-aspartate receptor-driven calcium influx potentiates the adverse effects of myocardial ischemia-reperfusion injury ex vivo. J Cardiovasc Pharmacol. 2017;70(5):329–38. https://doi.org/10.1097/FJC.0000000000000527.
Repas SJ, Saad NS, Janssen PML, Elnakish MT. Memantine, an NMDA receptor antagonist, prevents thyroxin-induced hypertension, but not cardiac remodeling. J Cardiovasc Pharmacol. 2017;70(5):305–13. https://doi.org/10.1097/FJC.0000000000000521.
Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008;295(2):H890–7. https://doi.org/10.1152/ajpheart.00099.2008.
Moshal KS, Kumar M, Tyagi N, Mishra PK, Metreveli N, Rodriguez WE, et al. Restoration of contractility in hyperhomocysteinemia by cardiac-specific deletion of NMDA-R1. Am J Physiol Heart Circ Physiol. 2009;296(3):H887–92. https://doi.org/10.1152/ajpheart.00750.2008.
Tyagi N, Vacek JC, Givvimani S, Sen U, Tyagi SC. Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia. J Recept Signal Transduct Res. 2010;30(2):78–87. https://doi.org/10.3109/10799891003614808.
Gao X, Xu X, Pang J, Zhang C, Ding JM, Peng X, et al. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol Res. 2007;56(5):559–69.
Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101(6):660–7. https://doi.org/10.1161/01.cir.101.6.660.
Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, et al. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86(6):692–9. https://doi.org/10.1161/01.res.86.6.692.
Miao W, Luo Z, Kitsis RN, Walsh K. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J Mol Cell Cardiol. 2000;32(12):2397–402. https://doi.org/10.1006/jmcc.2000.1283.
Kawamura S, Yoshida K, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-delta and -epsilon, which mediate functional protection in isolated rat heart. Am J Phys. 1998;275(6):H2266–71. https://doi.org/10.1152/ajpheart.1998.275.6.H2266.
Meng L, Zhang Z, Xu K, Qi G. HIV-1 gp120 induces autophagy in cardiomyocytes via the NMDA receptor. Int J Cardiol. 2013;167(6):2517–23. https://doi.org/10.1016/j.ijcard.2012.06.067.
Ma H, Chen SR, Chen H, Zhou JJ, Li DP, Pan HL. alpha2delta-1 couples to NMDA receptors in the hypothalamus to sustain sympathetic vasomotor activity in hypertension. J Physiol. 2018;596(17):4269–83. https://doi.org/10.1113/JP276394.
Lo WC, Lin HC, Ger LP, Tung CS, Tseng CJ. Cardiovascular effects of nitric oxide and N-methyl-D-aspartate receptors in the nucleus tractus solitarii of rats. Hypertension. 1997;30(6):1499–503. https://doi.org/10.1161/01.hyp.30.6.1499.
Sitniewska EM, Wisniewska RJ, Wisniewski K. The role of ionotropic receptors of glutaminic acid in cardiovascular system. A. the influence of ionotropic receptor NMDA agonist - 1R,3R-ACPD and antagonist - DL-AP7 on the systemic pressure in rats. Amino Acids. 2003;24(4):397–403. https://doi.org/10.1007/s00726-002-0342-4.
Huang CF, Su MJ. Positive inotropic action of NMDA receptor antagonist (+)-MK801 in rat heart. J Biomed Sci. 1999;6(6):387–98. https://doi.org/10.1007/bf02253670.
Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–66. https://doi.org/10.1016/S0140-6736(11)61454-2.
Ostermann M, Liu K. Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol. 2017;31(3):305–14. https://doi.org/10.1016/j.bpa.2017.09.001.
Leung JC, Marphis T, Craver RD, Silverstein DM. Altered NMDA receptor expression in renal toxicity: protection with a receptor antagonist. Kidney Int. 2004;66(1):167–76. https://doi.org/10.1111/j.1523-1755.2004.00718.x.
Mahieu S, Klug M, Millen N, Fabro A, Benmelej A, Contini MC. Monosodium glutamate intake affect the function of the kidney through NMDA receptor. Life Sci. 2016;149:114–9. https://doi.org/10.1016/j.lfs.2016.02.023.
Sharma A. Monosodium glutamate-induced oxidative kidney damage and possible mechanisms: a mini-review. J Biomed Sci. 2015;22(1):93. https://doi.org/10.1186/s12929-015-0192-5.
Pundir M, Arora S, Kaur T, Singh R, Singh AP. Effect of modulating the allosteric sites of N-methyl-D-aspartate receptors in ischemia-reperfusion induced acute kidney injury. J Surg Res. 2013;183(2):668–77. https://doi.org/10.1016/j.jss.2013.01.040.
Lin CS, Hung SF, Huang HS, Ma MC. Blockade of the N-methyl-D-aspartate glutamate receptor ameliorates lipopolysaccharide-induced renal insufficiency. PLoS One. 2015;10(7):e0132204. https://doi.org/10.1371/journal.pone.0132204.
Arora S, Kaur T, Kaur A, Singh AP. Glycine aggravates ischemia reperfusion-induced acute kidney injury through N-methyl-D-aspartate receptor activation in rats. Mol Cell Biochem. 2014;393(1–2):123–31. https://doi.org/10.1007/s11010-014-2052-0.
Szaroma W, Dziubek K, Kapusta E. Effect of N-methyl-D-aspartic acid on activity of superoxide dismutase, catalase, glutathione peroxidase and reduced glutathione level in selected organs of the mouse. Acta Physiol Hung. 2014;101(3):377–87. https://doi.org/10.1556/APhysiol.101.2014.003.
Albvr VR, Tan SH, Candasamy M, Bhattamisra SK. Diabetic nephropathy: An update on pathogenesis and drug development. Diabetes Metab Syndr. 2019;13(1):754–62. https://doi.org/10.1016/j.dsx.2018.11.054.
Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23(12):1917–28. https://doi.org/10.1681/ASN.2012040390.
Shen J, Wang R, He Z, Huang H, He X, Zhou J, et al. NMDA receptors participate in the progression of diabetic kidney disease by decreasing Cdc42-GTP activation in podocytes. J Pathol. 2016;240(2):149–60. https://doi.org/10.1002/path.4764.
Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T. Therapeutic potential of 20(S)-ginsenoside Rg(3) against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol. 2008;591(1–3):266–72. https://doi.org/10.1016/j.ejphar.2008.06.077.
Kundu S, Pushpakumar S, Sen U. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator. Nitric Oxide. 2015;46:172–85. https://doi.org/10.1016/j.niox.2015.02.003.
Melendez J, Liu M, Sampson L, Akunuru S, Han X, Vallance J, et al. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology. 2013;145(4):808–19. https://doi.org/10.1053/j.gastro.2013.06.021.
Kundu S, Pushpakumar SB, Tyagi A, Coley D, Sen U. Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am J Physiol Endocrinol Metab. 2013;304(12):E1365–78. https://doi.org/10.1152/ajpendo.00604.2012.
Leung JC, Ragland N, Marphis T, Silverstein DM. NMDA agonists and antagonists induce renal culture cell toxicity. Med Chem. 2008;4(6):565–71. https://doi.org/10.2174/157340608786242034.
Kaur A, Kaur T, Singh B, Pathak D, Singh Buttar H, Pal SA. Curcumin alleviates ischemia reperfusion-induced acute kidney injury through NMDA receptor antagonism in rats. Ren Fail. 2016;38(9):1462–7. https://doi.org/10.1080/0886022X.2016.1214892.
Singh AP, Singh N, Bedi PMS. Estradiol mitigates ischemia reperfusion-induced acute renal failure through NMDA receptor antagonism in rats. Mol Cell Biochem. 2017;434(1–2):33–40. https://doi.org/10.1007/s11010-017-3034-9.
Singh AP, Singh N, Bedi PM. Pioglitazone ameliorates renal ischemia reperfusion injury through NMDA receptor antagonism in rats. Mol Cell Biochem. 2016;417(1–2):111–8. https://doi.org/10.1007/s11010-016-2718-x.
Huang XT, Li C, Peng XP, Guo J, Yue SJ, Liu W, et al. An excessive increase in glutamate contributes to glucose-toxicity in beta-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci Rep. 2017;7:44120. https://doi.org/10.1038/srep44120.
Patterson S, Irwin N, Guo-Parke H, Moffett RC, Scullion SM, Flatt PR, et al. Evaluation of the role of N-methyl-D-aspartate (NMDA) receptors in insulin secreting beta-cells. Eur J Pharmacol. 2016;771:107–13. https://doi.org/10.1016/j.ejphar.2015.12.015.
Huang XT, Yue SJ, Li C, Huang YH, Cheng QM, Li XH, et al. A sustained activation of pancreatic NMDARs is a novel factor of beta-cell apoptosis and dysfunction. Endocrinology. 2017;158(11):3900–13. https://doi.org/10.1210/en.2017-00366.
Wollheim CB, Maechler P. Beta cell glutamate receptor antagonists: novel oral antidiabetic drugs? Nat Med. 2015;21(4):310–1. https://doi.org/10.1038/nm.3835.
Huang XT, Liu W, Zhou Y, Sun M, Sun CC, Zhang CY, et al. Endoplasmic reticulum stress contributes to NMDA-induced pancreatic beta-cell dysfunction in a CHOP-dependent manner. Life Sci. 2019;232:116612. https://doi.org/10.1016/j.lfs.2019.116612.
Otter S, Lammert E. Exciting times for pancreatic islets: glutamate signaling in endocrine cells. Trends Endocrinol Metab. 2016;27(3):177–88. https://doi.org/10.1016/j.tem.2015.12.004.
Boonnate P, Waraasawapati S, Hipkaeo W, Pethlert S, Sharma A, Selmi C, et al. Monosodium glutamate dietary consumption decreases pancreatic beta-cell mass in adult Wistar rats. PLoS One. 2015;10(6):e0131595. https://doi.org/10.1371/journal.pone.0131595.
Alberdi E, Sanchez-Gomez MV, Cavaliere F, Perez-Samartin A, Zugaza JL, Trullas R, et al. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47(3):264–72. https://doi.org/10.1016/j.ceca.2009.12.010.
Lechin F, van der Dijs B, Pardey-Maldonado B, Rivera JE, Lechin ME, Baez S. Amantadine reduces glucagon and enhances insulin secretion throughout the oral glucose tolerance test: central plus peripheral nervous system mechanisms. Diabetes Metab Syndr Obes. 2009;2:203–13. https://doi.org/10.2147/dmsott.s7606.
Marquard J, Stirban A, Schliess F, Sievers F, Welters A, Otter S, et al. Effects of dextromethorphan as add-on to sitagliptin on blood glucose and serum insulin concentrations in individuals with type 2 diabetes mellitus: a randomized, placebo-controlled, double-blinded, multiple crossover, single-dose clinical trial. Diabetes Obes Metab. 2016;18(1):100–3. https://doi.org/10.1111/dom.12576.
Seino S, Sugawara K, Yokoi N, Takahashi H. beta-Cell signalling and insulin secretagogues: a path for improved diabetes therapy. Diabetes Obes Metab. 2017;19(Suppl 1):22–9. https://doi.org/10.1111/dom.12995.
Arjona Ferreira JC, Corry D, Mogensen CE, Sloan L, Xu L, Golm GT, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis. 2013;61(4):579–87. https://doi.org/10.1053/j.ajkd.2012.11.043.
Huang XT, Yue SJ, Li C, Guo J, Huang YH, Han JZ, et al. Antenatal blockade of N-methyl-D-aspartate receptors by Memantine reduces the susceptibility to diabetes induced by a high-fat diet in rats with intrauterine growth restriction. Biol Reprod. 2017;96(5):960–70. https://doi.org/10.1095/biolreprod.116.145011.
Yabar CS, Winter JM. Pancreatic Cancer: a review. Gastroenterol Clin N Am. 2016;45(3):429–45. https://doi.org/10.1016/j.gtc.2016.04.003.
Malsy M, Gebhardt K, Gruber M, Wiese C, Graf B, Bundscherer A. Effects of ketamine, s-ketamine, and MK 801 on proliferation, apoptosis, and necrosis in pancreatic cancer cells. BMC Anesthesiol. 2015;15:111. https://doi.org/10.1186/s12871-015-0076-y.
North WG, Liu F, Lin LZ, Tian R, Akerman B. NMDA receptors are important regulators of pancreatic cancer and are potential targets for treatment. Clin Pharmacol. 2017;9:79–86. https://doi.org/10.2147/CPAA.S140057.
Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287(38):31666–73. https://doi.org/10.1074/jbc.R112.343061.
Chen X, Wu Q, You L, Chen S, Zhu M, Miao C. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur J Pharmacol. 2017;795:150–9. https://doi.org/10.1016/j.ejphar.2016.12.017.
Parbhu SK, Adler DG. Pancreatic neuroendocrine tumors: contemporary diagnosis and management. Hosp Pract (1995). 2016;44(3):109–19. https://doi.org/10.1080/21548331.2016.1210474.
Li L, Hanahan D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell. 2013;153(1):86–100. https://doi.org/10.1016/j.cell.2013.02.051.
Hanahan D. Herifogure formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315(6015):115–22. https://doi.org/10.1038/315115a0.
Robinson HPC, Li L. Autocrine, paracrine and necrotic NMDA receptor signalling in mouse pancreatic neuroendocrine tumour cells. Open Biol. 2017;7(12). https://doi.org/10.1098/rsob.170221.
Li L, Zeng Q, Bhutkar A, Galvan JA, Karamitopoulou E, Noordermeer D, et al. GKAP acts as a genetic modulator of NMDAR signaling to govern invasive tumor growth. Cancer Cell. 2018;33(4):736–51 e5. https://doi.org/10.1016/j.ccell.2018.02.011.
Francis ME, Eggers PW, Hostetter TH, Briggs JP. Association between serum homocysteine and markers of impaired kidney function in adults in the United States. Kidney Int. 2004;66(1):303–12. https://doi.org/10.1111/j.1523-1755.2004.00732.x.
Dalton ML, Gadson PF Jr, Wrenn RW, Rosenquist TH. Homocysteine signal cascade: production of phospholipids, activation of protein kinase C, and the induction of c-fos and c-myb in smooth muscle cells. FASEB J. 1997;11(8):703–11. https://doi.org/10.1096/fasebj.11.8.9240971.
Chen H, Fitzgerald R, Brown AT, Qureshi I, Breckenridge J, Kazi R, et al. Identification of a homocysteine receptor in the peripheral endothelium and its role in proliferation. J Vasc Surg. 2005;41(5):853–60. https://doi.org/10.1016/j.jvs.2005.02.021.
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–77. https://doi.org/10.1038/nrn3257.
Wejksza K, Rzeski W, Parada-Turska J, Zdzisinska B, Rejdak R, Kocki T, et al. Kynurenic acid production in cultured bovine aortic endothelial cells. Homocysteine is a potent inhibitor. Naunyn Schmiedeberg's Arch Pharmacol. 2004;369(3):300–4. https://doi.org/10.1007/s00210-004-0872-2.
Acknowledgements
We would like to thank Cheng Haipeng, Lan Jinrong, Fang Lijuan, Cao Yuanyuan, Li Yanghang, Luo Yongyu, Bao Xingwen, Hua Qingzhong, Liang Xinyue, and Qiu Yujia for their perpetual support and encouragement.
Funding
This study was funded by the National Natural Science Foundation of China (Grants 81170717, 81570065, 81870059).
Author information
Authors and Affiliations
Contributions
Feng Dandan and Ma Tianqi developed the conception of the work, Ma Tianqi drafted the manuscript, Cheng Qingmei contributed to the organization of tables and figures, Luo Ziqiang, Feng Dandan, and Chen Chen ensured that questions related to the accuracy or integrity of the work are appropriately investigated and resolved, and the authors were responsible for the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethic5al approval was not required for this review.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Ma, T., Cheng, Q., Chen, C. et al. Excessive Activation of NMDA Receptors in the Pathogenesis of Multiple Peripheral Organs via Mitochondrial Dysfunction, Oxidative Stress, and Inflammation. SN Compr. Clin. Med. 2, 551–569 (2020). https://doi.org/10.1007/s42399-020-00298-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00298-w