Abstract
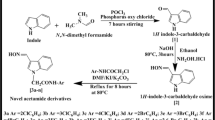
In the current research work, we report the synthesis of thiophenic derivatives 1–3 resulting in good yields (88–90%). This synthesis was undertaken in mild reaction conditions between readily available 2-amino-3-cyanothiophene, aldehydes and 2,5-dimethoxytetrahydrofuran. These compounds were brominated with NBS (N-bromosuccenimide) leading to new derivatives, namely 4–6 at room temperature for 24 h. In addition, we investigated the antioxidant activities using DPPH (2,2-diphenyl-1-picrylhydrazyl) and total ABTS (2,2’-azinobis-[3-ethylbenzthiazoline-6- sulfonic acid]) methods in order to evaluate the antioxidant activity of the new synthesized compounds. The results credibly indicate that compounds 1 and 3 showed the highest antioxidant activities. These new thiophenic compounds can be invested as substrates in the catalysis of C-H activation reaction.


Similar content being viewed by others
Abbreviations
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- ABTS:
-
2,2’-azinobis-[3-ethylbenzthiazoline-6- sulfonic acid
- NBS:
-
N-bromosuccinimide
- PBS:
-
Phosphate Buffer Saline
- TLC:
-
Thin Layer Chromatography
- NMR:
-
Nuclear Magnetic Resonance
- TE:
-
Trolox Equivalent
- IC50 :
-
inhibition concentration at 50%
References
Romagnoli R, Baraldi PG, Cara CL, Salvador MK, Preti D, Tabrizi MA, Balzarini J, Nussbaumer P, Bassetto M, Brancale A, Fu XH, Gao Y, Li J, Zhang SZ, Hamel E, Bortolozzi R, Basso G, Viola G (2014) Bioorg Med Chem 22:5097–5109. https://doi.org/10.1016/j.bmc.2013.12.030
Abdelhameid MK, Labib MB, Negmeldin AT, Al-Shorbagy M, Mohammed MR (2018) J Enzym Inhib Med Chem 33:1472–1493. https://doi.org/10.1080%2F14756366.2018.1503654
Zhao G, Alami M, Provot O (2017) RSC Adv 7:46007–46013. https://doi.org/10.1039/c7ra07340b. )
Bouzayani B, Ben Salem R, Soulé JF, Doucet H (2019) Eur J Org Chem 28:4581–4588. https://doi.org/10.1002/ejoc.201900775
Xu D, Li Z, Peng YX, Geng J, Qian HF, Huang W (2016) J Dye Pig 133:143–152. https://doi.org/10.1016/j.dyepig.2016.05.050
Li X, Li W, Ouyang M, Zhang Y, Wright DS, Zhang C (2017) J Mater Chem 5:12–28. https://doi.org/10.1039/C6TC04002K
Popowycz F, Métay E, Lemaire M (2011) Compt Rend Chem 14:621–628. https://doi.org/10.1016/j.crci.2010.06.006. )
Yagui J, Angel FA (2020) Opt Mat 109:110354–110359. https://doi.org/10.1016/j.optmat.2020.110354
Zhang Y, Xu J, Hu L, Guo T, He R, Yang W, Cao Y (2020) Org Elec 2020 81: 105670–105681. https://doi.org/10.1016/j.orgel.2020.105670
Guo BK, Yang F, Wang Y, Wei Q, Liu L, Zhong X, Wang L, Gong J, Li F, Wong W, Alamry KA, Zhao Y (2020) J Lumin 220:116963–117009. https://doi.org/10.1016/j.jlumin.2019.116963
Lugovik KI, Kanaa A, Benassi E, Belskaya NP (2021) As J Org Chem 10:400–411. https://doi.org/10.1002/ajoc.202000663
Archna PS, Chawla PA (2020) Bioorg Chem 101:104026. https://doi.org/10.1016/j.bioorg.2020.104026. )
Pathania S, Narang RR, Rawal RK (2019) Eur J Med Chem 180:486–508. https://doi.org/10.1016/j.ejmech.2019.07.043
Harit T, Bellaouchi R, Asehraou A, Rahal M, Bouabdallah I, Malek F (2017) J Mol Struct 1133:74–79. https://doi.org/10.1016/j.molstruc.2016.11.051. )
Yahia NM, Nahed AK, Seham A, Salem S, AlS, Thoraya AF, Mohammad SM (2017) Chem Cent J 11:75–85. https://doi.org/10.1186/s13065-017-0307-z
Nisheeth CD, Yogesh MR, Ashvinkumar GK, Keyur NS, Unnat PP, Vijay MN (2022) J Het Chemistry 59:75–87. https://doi.org/10.1002/ardp.200800026
Mishra P, Middha A, Saxena V, Saxena A (2016) J Pharm Biosci 4:64–68. https://doi.org/10.20510/ukjpb/4/i3/108388
Berrade L, Aisa B, Ramirez MJ, Galiano S, Guccione S, Moltzau LR, Levy FO, Nicoletti F, Battaglia G, Molinaro G, Aldana I, Monge A, Perez-Silanes S (2011) J Med Chem 54:3086–3090. https://doi.org/10.1021/jm2000773
Ashour HM, Shaabana OG, Rizk OH, El-Ashmawy IM (2013) Eur J Med Chem 62:341–351. https://doi.org/10.1016/j.ejmech.2012.12.003
Kang D, Ding X, Wu G, Huo Z, Zhou Z, Zhao T, Feng D, Wang Z, Tian Y, Daelemans D (2017) ACS Med Chem Lett 8:1188–1193. https://doi.org/10.1021/acsmedchemlett.7b00361. )
Shin-ichi K, Yuhei G, Takuya Y, Yuki S, Hiroyuki S, Yohsuke K, Atsuhiko I, Takashi D, Motohiro S, Toshio M, Akiya O, Tadayuki T (2018) J Molecules 23:885–909. https://doi.org/10.3390/molecules23040885
Maria A, Anaïs P-B, Nicolas PE B (2018) Med Chem Commun 5:759–782. https://doi.org/10.1039%2Fc7md00448f
Kang D, Fang Z, Huang B, Lu X, Zhang H, Xu H, Huo Z, Zhou Z, Yu Z, Meng Q, Wu G, Ding X, Tian Y, Daelemans D, Clercq ED, Pannecouque C, Zhan P, Liu X (2017) J Med Chem 60:4424–4443. https://doi.org/10.1021/acs.jmedchem.7b00332
Rashmi S, Prabhakar KV (2019) BMC Chem 13:54–66. https://doi.org/10.1186/s13065-019-0569-8
Kotaiah Y, Harikrishana N, Nagaraju K, Rao V (2012) Eur J Med Chem 58:340–345. https://doi.org/10.1016/j.ejmech.2012.10.007
Rayssa MDdaC, Renaud Z, Sarah B, Msc RMD, da José C, Jean-Luc PS-J, Marie-Paule D, Francisco M-L JBM-J (2020) Chem Med Chem 8:716–725. https://doi.org/10.1002/cmdc.201900688
Mohamed EK (2020) Synth Com 17:2590–2616. https://doi.org/10.1080/00397911.2020.1777311
Luna IS, Neves WW, de Lima-Neto RG, Albuquerque APB, Pitta MGR, Rêgo MJBM, Neves RP, Scotti MT, Mendonça-Junior FJB (2021) J Braz Chem 32:1017–1029. https://doi.org/10.21577/0103-5053.20210004
Dufresnea S, Skene WG (2012) J Phys Org Chem 25:211–221. https://doi.org/10.1002/poc.1894
Smari I, Youssef C, Ben Ammar H, Ben Hassine B, Soule JF, Doucet H (2015) Tetrahedron 71:6586–6593. https://doi.org/10.1016/j.tet.2015.03.022
Khedher O, Rigane G, Ben Salem R, Moussaoui Y (2020) Chem Afri. https://doi.org/10.1007/s42250-020-00170-3
Elakremi M, Sillero L, Ayed L, Mannai F, Ben Salem R, Labidi J, Moussaoui Y (2022) Cell Chem Technol 56:309–319. https://doi.org/10.35812/CelluloseChemTechnol.2022.56.27
Gendron D, Vamvounis G (2015) J Org Synt 47:385–414. https://doi.org/10.1080/00304948.2015.1088752
Tamilavan V, Cho N, Kim C, Ko J, Hyun MH (2012) Tetrahedron 68:5890–5897. https://doi.org/10.1016/j.tet.2012.04.104
Qu Z, Jiang Z, Chen L, Xiao D, Tian C, Gao W, Wei Q, Gong J (2012) Appl Polym Sci 124:1186–1192. https://doi.org/10.1002/app.35078
Pati PB, Zade SS (2014) J RSC Adv 4:17022–17027. https://doi.org/10.1039/c4ra01993h
Mabkhot YN, Barakat A, Al-Majid AM, Alshahrani S, Yousuf S, Choudhary MI (2013) J Cent Chem 7:112–120. https://doi.org/10.1186/1752-153X-7-112
Acknowledgements
All Authors are extremely grateful to scientific Ministry of Higher Education Research of Tunisia for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of Interest. Ridha Ben Salem and Ghayth Rigane are Associate Editors of Chemistry Africa.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouzayani, B., Elakermi, M., Mosbah, M.B. et al. Synthesis and Antioxidant Assessment of some Derived Compounds from 2-Amino-3-Cyanothiophene. Chemistry Africa 6, 1201–1207 (2023). https://doi.org/10.1007/s42250-022-00559-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00559-2