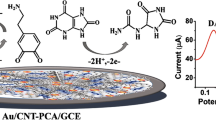
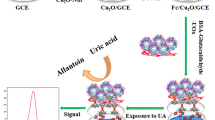
Abstract
Efficient uric acid (UA) detection from the blood serum is a key indicator of overall health status and thus helps in public health monitoring. Hence, in this article, we have proposed a non-enzymatic UA sensor using a novel polypyrrole-carbon black-Co3O4 (PPy-CB-Co3O4) nanocomposite (NC) modified glassy carbon electrode. Modern analytical tools like FE-SEM, TEM, EDXS, XRD, XPS, and FTIR spectroscopy were used to characterize the PPy-CB-Co3O4 nanocomposite. XRD and XPS analysis confirmed the fruitful development of nanocomposite consisting of PPy-CB and Co3O4. TEM images revealed that Co3O4 nanoparticles (NPs) were randomly dispersed on the PPy-CB sheets. In the electrochemical investigations, PPy-CB-Co3O4/GCE sensor showed excellent sensitivity (0.8786 μA μM−1 cm−2), wide LDR (0.75–305 μM) to cover the entire UA range in human blood serum, and extremely lower detection limit (LOD ~ 0.46 μM). The newly developed UA sensor was further used to check the potential chemical interference using several biomolecules, presenting an extreme selectivity in UA detection. The PPy-CB-Co3O4/GCE sensor also exhibited satisfactory results in detecting UA levels in human blood serum. In UA determination, the PPy-CB-Co3O4/GCE sensor also displayed excellent reproducibility, repeatability, and stability. It is anticipated that this PPy-CB-Co3O4 nanocomposite fabricated GCE will emerge as an effective route to develop an efficient non-enzymatic UA sensor.









Similar content being viewed by others
References
Huang W, Cao Y, Chen Y, Zhou Y, Huang Q (2015) 3-D periodic mesoporous nickel oxide for nonenzymatic uric acid sensors with improved sensitivity. Appl Surf Sci 359:221–226. https://doi.org/10.1016/j.apsusc.2015.10.028
Qi S, Zhao B, Tang H, Jiang X (2015) Determination of ascorbic acid, dopamine, and uric acid by a novel electrochemical sensor based on pristine graphene. Electrochim Acta 161:395–402. https://doi.org/10.1016/j.electacta.2015.02.116
Rahman MM, Ahmed J, Asiri AM (2017) A glassy carbon electrode modified with γ-Ce2S3-decorated CNT nanocomposites for uric acid sensor development: a real sample analysis. RSC Adv 7:14649–14659. https://doi.org/10.1039/c6ra27414e
Erden PE, Kiliç E (2013) A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 107:312–323. https://doi.org/10.1016/j.talanta.2013.01.043
Jindal K, Tomar M, Gupta V (2013) Nitrogen-doped zinc oxide thin films biosensor for determination of uric acid. Analyst 138:4353–4362. https://doi.org/10.1039/c3an36695b
Luo L, Li F, Zhu L, Ding Y, Zhang Z, Deng D et al (2012) Simultaneous determination of epinephrine and uric acid at ordered mesoporous carbon modified glassy carbon electrode. Anal Methods 4:2417–2422. https://doi.org/10.1039/c2ay25168j
Xu P, Li R, Tu Y, Yan J (2015) A gold nanocluster-based sensor for sensitive uric acid detection. Talanta 144:704–709. https://doi.org/10.1016/j.talanta.2015.07.027
Ahmed J, Faisal M, Harraz FA, Jalalah M, Alsareii SA (2021) Porous silicon-mesoporous carbon nanocomposite based electrochemical sensor for sensitive and selective detection of ascorbic acid in real samples. J Taiwan Inst Chem Eng 125:360–371. https://doi.org/10.1016/j.jtice.2021.06.018
Ahmed J, Rashed A, Faisal M, Harraz FA, Jalalah M, Alsareii SA (2021) Novel SWCNTs-mesoporous silicon nanocomposite as efficient non-enzymatic glucose biosensor. Appl Surf Sci 552:149477. https://doi.org/10.1016/j.apsusc.2021.149477
Rahman MM, Ahmed J, Asiri AM (2017) Development of creatine sensor based on antimony-doped tin oxide (ATO) nanoparticles. Sensors Actuators, B Chem 242:167–175. https://doi.org/10.1016/j.snb.2016.11.053
Rahman MM, Ahmed J, Asiri AM (2019) Selective bilirubin sensor fabrication based on doped IAO nanorods for environmental remediation. New J Chem 43:19298–19307. https://doi.org/10.1039/c9nj05477d
Karimi-Maleh H, Orooji Y, Karimi F, Alizadeh M, Baghayeri M, Rouhi J et al (2021) A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens Bioelectron 184:113252. https://doi.org/10.1016/j.bios.2021.113252
Deshmukh MA, Kang B-C, Ha T-J (2020) Non-enzymatic electrochemical glucose sensors based on polyaniline/reduced-graphene-oxide nanocomposites functionalized with silver nanoparticles. J Mater Chem C 8:5112–5123. https://doi.org/10.1039/C9TC06836H
Garkani Nejad F, Tajik S, Beitollahi H, Sheikhshoaie I (2021) Magnetic nanomaterials based electrochemical (bio)sensors for food analysis. Talanta 228:122075. https://doi.org/10.1016/j.talanta.2020.122075
Li F, Zhang B, Dong S, Wang E (1997) A novel method of electrodepositing highly dispersed nano palladium particles on glassy carbon electrode. Electrochim Acta 42:2563–2568. https://doi.org/10.1016/S0013-4686(96)00450-1
Oukil D, Benhaddad L, Aitout R, Makhloufi L, Pillier F, Saidani B (2014) Electrochemical synthesis of polypyrrole films doped by ferrocyanide ions onto iron substrate: application in the electroanalytical determination of uric acid. Sensors Actuators, B Chem 204:203–210. https://doi.org/10.1016/j.snb.2014.07.086
Wang F, Chi C, Yu B, Ye B (2015) Simultaneous voltammetric determination of dopamine and uric acid based on Langmuir–Blodgett film of calixarene modified glassy carbon electrode. Sensors Actuators B Chem 221:1586–93. https://doi.org/10.1016/j.snb.2015.06.155
Cai W, Lai J, Lai T, Xie H, Ye J (2016) Controlled functionalization of flexible graphene fibers for the simultaneous determination of ascorbic acid, dopamine and uric acid. Sensors Actuators, B Chem 224:225–232. https://doi.org/10.1016/j.snb.2015.09.079
Temerk Y, Ibrahim H (2016) A new sensor based on in doped CeO2 nanoparticles modified glassy carbon paste electrode for sensitive determination of uric acid in biological fluids. Sensors Actuators, B Chem 224:868–877. https://doi.org/10.1016/j.snb.2015.11.029
Bhakta AK, Mascarenhas RJ, D’Souza OJ, Satpati AK, Detriche S, Mekhalif Z et al (2015) Iron nanoparticles decorated multi-wall carbon nanotubes modified carbon paste electrode as an electrochemical sensor for the simultaneous determination of uric acid in the presence of ascorbic acid, dopamine and L-tyrosine. Mater Sci Eng C Mater Biol Appl 57:328–337. https://doi.org/10.1016/j.msec.2015.08.003
Tajik S, Orooji Y, Ghazanfari Z, Karimi F, Beitollahi H, Varma RS et al (2021) Nanomaterials modified electrodes for electrochemical detection of Sudan I in food. J Food Meas Charact 15:3837–3852. https://doi.org/10.1007/s11694-021-00955-1
Rashed MA, Faisal M, Harraz FA, Jalalah M, Alsaiari M (2020) Al-Assiri MS (2020) rGO/ZnO/Nafion nanocomposite as highly sensitive and selective amperometric sensor for detecting nitrite ions (NO2−). J Taiwan Inst Chem Eng 112:345–356. https://doi.org/10.1016/j.jtice.2020.05.015
Davoudi S, Givianrad MH, Saber-Tehrani M, Azar PA (2019) A novel electrochemical sensor based on Co3O4-CeO2-ZnO multi metal oxide nanocomposite for simultaneous detection of nanomolar Pb2+ and Hg2+ in different kind of spices. Indian J Chem - Sect A Inorganic, Phys Theor Anal Chem 58A:1075–1084
Khand NH, Palabiyik IM, Buledi JA, Ameen S, Memon AF, Ghumro T et al (2021) Functional Co3O4 nanostructure-based electrochemical sensor for direct determination of ascorbic acid in pharmaceutical samples. J Nanostructure Chem 11:455–468. https://doi.org/10.1007/s40097-020-00380-8
Luo J, Wu J, Liu Z, Li Z, Deng L (2019) Controlled synthesis of porous Co3O4 nanostructures for efficient electrochemical sensing of glucose. J Nanomater 2019:8346251. https://doi.org/10.1155/2019/8346251
Xu J-L, Dai R-X, Xin Y, Sun Y-L, Li X, Yu Y-X et al (2017) Efficient and reversible electron doping of semiconductor-enriched single-walled carbon nanotubes by using decamethylcobaltocene. Sci Rep 7:6751. https://doi.org/10.1038/s41598-017-05967-w
Wang M, Shi M, Meng E, Gong F, Li F (2020) Non-enzymatic glucose sensor based on three-dimensional hierarchical Co3O4 nanobooks. Micro Nano Lett 15:193–197. https://doi.org/10.1049/mnl.2019.0552
Du J, Tao Y, Zhang J, Xiong Z, Xie A, Luo S et al (2019) Co3O4-CuNi/reduced graphene composite for non-enzymatic detection of ascorbic acid. Mater Technol 34:665–673. https://doi.org/10.1080/10667857.2019.1612551
Aflatoonian MR, Tajik S, Aflatoonian B, Beitollahi H, Zhang K, Van LQ et al (2020) A screen-printed electrode modified with graphene/Co3O4 nanocomposite for electrochemical detection of tramadol. Front Chem 8:1–8. https://doi.org/10.3389/fchem.2020.562308
Zhe T, Li M, Li F, Li R, Bai F, Bu T, Jia P, Wang L (2022) Integrating electrochemical sensor based on MoO3/Co3O4 heterostructure for highly sensitive sensing of nitrite in sausages and water Food Chem 367:130666. https://doi.org/10.1016/j.foodchem.2021.130666
Qi Y, Cao Y, Meng X, Cao J, Li X, Hao Q et al (2019) Facile synthesis of 3D sulfur/nitrogen co-doped graphene derived from graphene oxide hydrogel and the simultaneous determination of hydroquinone and catechol. Sensors Actuators, B Chem 279:170–176. https://doi.org/10.1016/j.snb.2018.09.067
Nejad FG, Beitollahi H, Tajik S, Jahani S (2019) La3+ -doped Co3O4 nanoflowers modified graphite screen printed electrode for electrochemical sensing of vitamin B6. Anal Bioanal Chem Res 6:69–79. https://doi.org/10.22036/abcr.2018.134444.1212
Harraz FA, Salem MS, Sakka T, Ogata YH (2008) Hybrid nanostructure of polypyrrole and porous silicon prepared by galvanostatic technique. Electrochim Acta 53:3734–3740. https://doi.org/10.1016/j.electacta.2007.09.019
Wei H, Li A, Kong D, Li Z, Cui D, Li T et al (2021) Polypyrrole/reduced graphene aerogel film for wearable piezoresisitic sensors with high sensing performances. Adv Compos Hybrid Mater 4:86–95. https://doi.org/10.1007/s42114-020-00201-0
Tigari G, Manjunatha JG, Raril C, Hareesha N (2019) Determination of riboflavin at carbon nanotube paste electrodes modified with an anionic surfactant. ChemistrySelect 4:2168–2173. https://doi.org/10.1002/slct.201803191
Mashhadizadeh MH, Kalantarian SM, Azhdeh A (2021) A novel electrochemical sensor for simultaneous determination of hydroquinone, catechol, and resorcinol using a carbon paste electrode modified by Zn-MOF, nitrogen-doped graphite, and AuNPs. Electroanalysis 33:160–169. https://doi.org/10.1002/elan.202060326
Raril C, Manjunatha JG (2020) A simple approach for the electrochemical determination of vanillin at ionic surfactant modified graphene paste electrode. Microchem J 154:104575. https://doi.org/10.1016/j.microc.2019.104575
Goto T, Hyodo T, Ueda T, Kamada K, Kaneyasu K, Shimizu Y (2015) CO-sensing properties of potentiometric gas sensors using an anion-conducting polymer electrolyte and Au-loaded metal oxide electrodes. Electrochim Acta 166:232–243. https://doi.org/10.1016/j.electacta.2015.03.045
Naveen MH, Gurudatt NG, Shim YB (2017) Applications of conducting polymer composites to electrochemical sensors: A review. Appl Mater Today 9:419–433. https://doi.org/10.1016/j.apmt.2017.09.001
Harraz FA (2014) Electrochemical formation of a novel porous silicon/polypyrrole hybrid structure with enhanced electrical and optical characteristics. J Electroanal Chem 729:68–74. https://doi.org/10.1016/j.jelechem.2014.07.015
Sachdeva S, Koper SJH, Sabetghadam A, Soccol D, Gravesteijn DJ, Kapteijn F et al (2017) Gas phase sensing of alcohols by metal organic framework-polymer composite materials. ACS Appl Mater Interfaces 9:24926–24935. https://doi.org/10.1021/acsami.7b02630
Ahmed J, Faisal M, Harraz FA, Jalalah M, Alsareii SA (2022) Development of an amperometric biosensor for dopamine using novel mesoporous silicon nanoparticles fabricated via a facile stain etching approach. Physica E 135:114952. https://doi.org/10.1016/j.physe.2021.114952
Umar A, Kumar R, Algadi H, Ahmed J, Jalalah M, Ibrahim AA et al (2021) Highly sensitive and selective 2-nitroaniline chemical sensor based on Ce-doped SnO2 nanosheets/Nafion-modified glassy carbon electrode. Adv Compos Hybrid Mater 2021. https://doi.org/10.1007/s42114-021-00283-4
Abdullah MM, Faisal M, Ahmed J, Harraz FA, Jalalah M, Alsareii SA (2021) Sensitive detection of aqueous methanol by electrochemical route using mesoporous α-Fe2O3 doped CdSe nanostructures modified glassy carbon electrode. J Electrochem Soc 168:057525. https://doi.org/10.1149/1945-7111/ac0175
Rahman MM, Ahmed J, Asiri AM, Alamry KA (2020) Fabrication of a hydrazine chemical sensor based on facile synthesis of doped NZO nanostructure materials. New J Chem 44:13018–13029. https://doi.org/10.1039/d0nj02719g
Subhan MA, Chandra Saha P, Ahmed J, Asiri AM, Al-Mamun M, Rahman MM (2020) Development of an ultra-sensitive para -nitrophenol sensor using tri-metallic oxide MoO2.Fe3O4.CuO nanocomposites. Mater Adv 1:2831–9. https://doi.org/10.1039/d0ma00629g
Aqlan FM, Alam MM, Al-Bogami AS, Saleh TS, Wani MY, Al-Farga A et al (2021) Efficient electro-chemical sensor for sensitive Cd2+ detection based on novel in-situ synthesized hydrazonoyl bromide (HB). J Mol Struct 1231:129690. https://doi.org/10.1016/j.molstruc.2020.129690
Ahmed J, Faisal M, Jalalah M, Alsaiari M, Alsareii SA, Harraz FA (2021) An efficient amperometric catechol sensor based on novel polypyrrole-carbon black doped α-Fe2O3 nanocomposite. Colloids Surfaces A Physicochem Eng Asp 619:126469. https://doi.org/10.1016/j.colsurfa.2021.126469
Liu J, Jin R, Qiao Y, Wu Y, Wang X, Wang Y (2018) Determination of Lead(II) using glassy carbon electrode modified with hexagonal Co3O4 microparticles. Int J Electrochem Sci 13:10415–26. https://doi.org/10.20964/2018.11.45
Bhargava R, Khan S, Ahmad N, Ansari MMN (2018) Investigation of structural, optical and electrical properties of Co3O4 nanoparticles. AIP Conf Proc 1953:1–5. https://doi.org/10.1063/1.5032369
Xiao L, Qi H, Qu K, Shi C, Cheng Y, Sun Z et al (2021) Layer-by-layer assembled free-standing and flexible nanocellulose/porous Co3O4 polyhedron hybrid film as supercapacitor electrodes. Adv Compos Hybrid Mater 4:306–316. https://doi.org/10.1007/s42114-021-00223-2
Xie P, Liu Y, Feng M, Niu M, Liu C, Wu N et al (2021) Hierarchically porous Co/C nanocomposites for ultralight high-performance microwave absorption. Adv Compos Hybrid Mater 4:173–185. https://doi.org/10.1007/s42114-020-00202-z
Zhang DX, Yoshikawa C, Welch NG, Pasic P, Thissen H, Voelcker NH (2019) Spatially controlled surface modification of porous silicon for sustained drug delivery applications. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-018-37750-w
Zhou M, Zhu L, Cao Y, Zhao R, Qian J, Ai X et al (2012) Fe(CN)6–4-doped polypyrrole: a high-capacity and high-rate cathode material for sodium-ion batteries. RSC Adv 2:5495–5498. https://doi.org/10.1039/c2ra20666h
Cao J, Wang Y, Chen J, Li X, Walsh FC, Ouyang JH et al (2015) Three-dimensional graphene oxide/polypyrrole composite electrodes fabricated by one-step electrodeposition for high performance supercapacitors. J Mater Chem A 3:14445–14457. https://doi.org/10.1039/c5ta02920a
Abu-Zied BM, Alam MM, Asiri AM, Ahmed J, Rahman MM (2020) Efficient hydroquinone sensor development based on Co3O4 nanoparticle. Microchem J 157:104972. https://doi.org/10.1016/j.microc.104972
Mujtaba J, Sun H, Huang G, Mølhave K, Liu Y, Zhao Y et al (2016) Nanoparticle decorated ultrathin porous nanosheets as hierarchical Co3O4 nanostructures for lithium ion battery anode materials. Sci Rep 6:1–8. https://doi.org/10.1038/srep20592
Abu-Zied BM (2019) A novel foam combustion approach for the synthesis of nano-crystalline cobalt oxide powder. Ceram Int 45:4540–4548. https://doi.org/10.1016/j.ceramint.2018.11.140
Abu-Zied BM, Alamry KA (2019) Green synthesis of 3D hierarchical nanostructured Co3O4/carbon catalysts for the application in sodium borohydride hydrolysis. J Alloys Compd 798:820–831. https://doi.org/10.1016/j.jallcom.2019.05.249
Abu-Zied BM, Alam MM, Asiri AM, Schwieger W, Rahman MM (2019) Fabrication of 1,2-dichlorobenzene sensor based on mesoporous MCM-41 material. Colloids Surfaces A Physicochem Eng Asp 562:161–169. https://doi.org/10.1016/j.colsurfa.2018.11.024
Duguet T, Amin-Chalhoub E, Samélor D, Pugliara A, Vahlas C (2018) Black Co oxides coatings for thermosensitive polymer surfaces by low-temperature DLI-MOCVD. Surf Coatings Technol 349:941–948. https://doi.org/10.1016/j.surfcoat.2018.05.087
He Q, Tian Y, Wu Y, Liu J, Li G, Deng P et al (2019) Facile and ultrasensitive determination of 4-nitrophenol based on acetylene black paste and graphene hybrid electrode. Nanomater (Basel, Switzerland) 9(3):429. https://doi.org/10.3390/nano9030429
Kaur D, Bagga V, Behera N, Thakral B, Asija A, Kaur J et al (2019) SnSe/SnO2 nanocomposites: novel material for photocatalytic degradation of industrial waste dyes. Adv Compos Hybrid Mater 2:763–776. https://doi.org/10.1007/s42114-019-00130-7
Danish M, Qamar M, Suliman M, Muneer M (2020) Photoelectrochemical and photocatalytic properties of Fe@ZnSQDs/TiO2 nanocomposites for degradation of different chromophoric organic pollutants in aqueous suspension. Adv Compos Hybrid Mater 3:570–582. https://doi.org/10.1007/s42114-020-00187-9
Xu H, Hai Z, Diwu J, Zhang Q, Gao L, Cui D et al (2015) Synthesis and microwave absorption properties of core-shell structured Co3O4-PANI nanocomposites. J Nanomater 2015:845983. https://doi.org/10.1155/2015/845983
Gongalsky MB, Kargina JV, Cruz JF, Sánchez-Royo JF, Chirvony VS, Osminkina LA et al (2019) Formation of Si/SiO2 luminescent quantum dots from mesoporous silicon by sodium tetraborate/citric acid oxidation treatment. Front Chem 7:165. https://doi.org/10.3389/fchem.2019.00165
Xu M, Huang Y, Chen R, Huang Q, Yang Y, Zhong L et al (2021) Green conversion of Ganoderma lucidum residues to electrode materials for supercapacitors. Adv Compos Hybrid Mater 2021. https://doi.org/10.1007/s42114-021-00271-8
Yu L, Zhang P, Dai H, Chen L, Ma H, Lin M et al (2017) An electrochemical sensor based on Co3O4 nanosheets for lead ions determination. RSC Adv 7:39611–39616. https://doi.org/10.1039/c7ra06269a
Zhang J, Shu D, Zhang T, Chen H, Zhao H, Wang Y et al (2012) Capacitive properties of PANI/MnO2 synthesized via simultaneous-oxidation route. J Alloys Compd 532:1–9. https://doi.org/10.1016/j.jallcom.2012.04.006
Harraz FA, Faisal M, Al-Salami AE, El-Toni AM, Almadiy AA, Al-Sayari SA et al (2019) Silver nanoparticles decorated stain-etched mesoporous silicon for sensitive, selective detection of ascorbic acid. Mater Lett 234:96–100. https://doi.org/10.1016/j.matlet.2018.09.076
Wei H, Gu H, Guo J, Cui D, Yan X, Liu J et al (2018) Significantly enhanced energy density of magnetite/polypyrrole nanocomposite capacitors at high rates by low magnetic fields. Adv Compos Hybrid Mater 1:127–134. https://doi.org/10.1007/s42114-017-0003-4
Qiao G, Wang S, Wang X, Chen X, Wang X, Cui H (2021) Ni/Co/black phosphorus nanocomposites for Q235 carbon steel corrosion-resistant coating. Adv Compos Hybrid Mater 2021. https://doi.org/10.1007/s42114-021-00268-3
Hsine Z, Blili S, Milka R, Dorizon H, Said AH, Korri-Youssoufi H (2020) Sensor based on redox conjugated poly(para-phenylene) for the simultaneous detection of dopamine, ascorbic acid, and uric acid in human serum sample. Anal Bioanal Chem 412:4433–4446. https://doi.org/10.1007/s00216-020-02686-6
Tajik S, Beitollahi H, Jang HW, Shokouhimehr M (2021) A screen printed electrode modified with Fe3O4@polypyrrole-Pt core-shell nanoparticles for electrochemical detection of 6-mercaptopurine and 6-thioguanine. Talanta 232:122379. https://doi.org/10.1016/j.talanta.2021.122379
Khalilzadeh MA, Tajik S, Beitollahi H, Venditti RA (2020) Green synthesis of magnetic nanocomposite with iron oxide deposited on cellulose nanocrystals with copper (Fe3O4@CNC/Cu): Investigation of catalytic activity for the development of a venlafaxine electrochemical sensor. Ind Eng Chem Res 59(10):4219–4228. https://doi.org/10.1021/acs.iecr.9b06214
Fotouhi L, Fatollahzadeh M, Heravi MM (2012) Electrochemical behavior and voltammetric determination of sulfaguanidine at a glassy carbon electrode modified with a multi-walled carbon nanotube. Int J Electrochem Sci 7(5):3919–3928
Abrori SA, Septiani NLW, Hakim FN, Maulana A, Suyatman N et al (2021) Non-enzymatic electrochemical detection for uric acid based on a glassy carbon electrode modified with MOF-71. IEEE Sens J 21:170–177. https://doi.org/10.1109/JSEN.2020.3014298
Ahmed J, Faisal M, Jalalah M, Alsareii SA, Harraz FA (2021) Novel polypyrrole-carbon black doped ZnO nanocomposite for efficient amperometric detection of hydroquinone. J Electroanal Chem 898:115631. https://doi.org/10.1016/j.jelechem.2021.115631
Vladimirova S, Krivetskiy V, Rumyantseva M, Gaskov A, Mordvinova N, Lebedev O et al (2017) Co3O4 as p-type material for CO sensing in humid air. Sensors (Switzerland) 17:1–13. https://doi.org/10.3390/s17102216
Crosnier de Lassichere C, Latapie L, Evrard D, Gros P (2018) New insight into the EC’ mechanism of uric acid regeneration in the presence of ascorbic acid on a poly(3,4-ethylenedioxithiophene) modified gold electrode. Electroanalysis 30:1645–1650. https://doi.org/10.1002/elan.201800024
Khan MMI, Haque AMJ, Kim K (2013) Electrochemical determination of uric acid in the presence of ascorbic acid on electrochemically reduced graphene oxide modified electrode. J Electroanal Chem 700:54–59. https://doi.org/10.1016/j.jelechem.2013.04.014
Pessoa J, Sárkány Z, Ferreira-da-Silva F, Martins S, Almeida MR, Li J et al (2010) Functional characterization of Arabidopsis thaliana transthyretin-like protein. BMC Plant Biol 10:30. https://doi.org/10.1186/1471-2229-10-30
Buledi JA, Ameen S, Memon SA, Fatima A, Solangi AR, Mallah A et al (2021) An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid. Open Chem 19:481–491. https://doi.org/10.1515/chem-2021-0029
Piedras J, Dominguez RB, Gutiérrez JM (2021) Determination of uric acid in artificial saliva with compact AMP3291 reader and au nanoparticles modified electrode. Chemosensors 9:1–11. https://doi.org/10.3390/chemosensors9040073
Abbas MW, Soomro RA, Kalwar NH, Zahoor M, Avci A, Pehlivan E et al (2019) Carbon quantum dot coated Fe3O4 hybrid composites for sensitive electrochemical detection of uric acid. Microchem J 146:517–524. https://doi.org/10.1016/j.microc.2019.01.034
Lv J, Li C, Feng S, Chen SM, Ding Y, Chen C et al (2019) A novel electrochemical sensor for uric acid detection based on PCN/MWCNT. Ionics (Kiel) 25:4437–4445. https://doi.org/10.1007/s11581-019-03010-8
Sun H, Chao J, Zuo X, Su S, Liu X, Yuwen L et al (2014) Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv 4:27625–27629. https://doi.org/10.1039/c4ra04046e
Shi Y, Wang J, Li S, Yan B, Xu H, Zhang K et al (2017) The enhanced photo-electrochemical detection of uric acid on Au nanoparticles modified glassy carbon electrode. Nanoscale Res Lett 12 6. https://doi.org/10.1186/s11671-017-2225-3
Kumar SS, Mathiyarasu J, Phani KL, Jain YK, Yegnaraman V (2005) Determination of uric acid in the presence of ascorbic acid using poly(3,4-ethylenedioxythiophene)-modified electrodes. Electroanalysis 17:2281–2286. https://doi.org/10.1002/elan.200503375
Noroozifar M, Khorasani-Motlagh M, Taheri A (2010) Preparation of silver hexacyanoferrate nanoparticles and its application for the simultaneous determination of ascorbic acid, dopamine and uric acid. Talanta 80:1657–1664. https://doi.org/10.1016/j.talanta.2009.10.005
Ahmed J, Rahman MM, Siddiquey IA, Asiri AM, Hasnat MA (2018) Efficient hydroquinone sensor based on zinc, strontium and nickel based ternary metal oxide (TMO) composites by differential pulse voltammetry. Sensors Actuators, B Chem 256:383–392. https://doi.org/10.1016/j.snb.2017.10.076
Rahman MM, Ahmed J (2018) Cd-doped Sb2O4 nanostructures modified glassy carbon electrode for efficient detection of melamine by electrochemical approach. Biosens Bioelectron 102:631–636. https://doi.org/10.1016/j.bios.2017.12.007
Ahmed J, Rahman MM, Siddiquey IA, Asiri AM, Hasnat MA (2017) Efficient Bisphenol-A detection based on the ternary metal oxide (TMO) composite by electrochemical approaches. Electrochim Acta 246:597–605. https://doi.org/10.1016/j.electacta.2017.06.072
Rahman MM, Ahmed J, Asiri AM, Siddiquey IA, Hasnat MA (2016) Development of highly-sensitive hydrazine sensor based on facile CoS2-CNT nanocomposites. RSC Adv 6:90470–90479. https://doi.org/10.1039/c6ra08772h
Rahman MM, Ahmed J, Asiri AM (2018) Thiourea sensor development based on hydrothermally prepared CMO nanoparticles for environmental safety. Biosens Bioelectron 99:586–592. https://doi.org/10.1016/j.bios.2017.08.039
Acknowledgements
The authors are thankful to the Deanship of Scientific Research at Najran University, Kingdom of Saudi Arabia for funding this work through a Grant: Research code NU/-/SERC/10/523. We also acknowledge Najran University Hospital for providing human blood serum samples. Jahir Ahmed acknowledges support from the Research and Development Office, the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, in cooperation with Najran University in the form of a postdoctoral fellowship.
Funding
This research was supported by the Deanship of Scientific Research at Najran University, Kingdom of Saudi Arabia through a Grant: Research code NU/-/SERC/10/523.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, J., Faisal, M., Alsareii, S.A. et al. Highly sensitive and selective non-enzymatic uric acid electrochemical sensor based on novel polypyrrole-carbon black-Co3O4 nanocomposite. Adv Compos Hybrid Mater 5, 920–933 (2022). https://doi.org/10.1007/s42114-021-00391-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-021-00391-1