Abstract
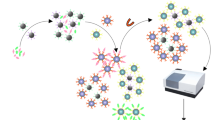
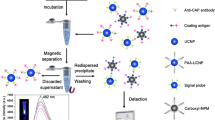
Some veterinary drug residues in food products and environment have been widely regarded as severe threats to human health. Rapid and simultaneous detection methods are crucial to monitor and control veterinary drug usage. Here, we propose a fluorescence biosensor utilizing immunomagnetic beads (IMBs) and quantum dots (QDs) for the rapid and simultaneous detection of 1-adamantylamine (ADA), enrofloxacin (ENR) and tilmicosin (TIL) in raw chicken meat. A pretreatment method using sodium phosphotungstate–magnesium as extraction reagent was developed to simultaneously extract ADA, ENR and TIL from chicken meat with minor interference in background or response. By adding the IMBs modified with three types of antibodies and the QD-antigens modified with three types of BSA-antigens to sample, IMBs competitively conjugated to target antigens in a sample or QD-antigens. After magnetic separation, the residual QD-antigens were adopted to collect signals using fluorescence spectroscopy. Using QDs with well separated emission peaks, the detection of one type of targets was minorly interfered by the others. Under the optimum conditions, the biosensor exhibited the limit of detection of 0.96, 3.32, and 3.17 ng/mL for ADA, ENR and TIL in chicken samples, respectively, as well as good specificity. Due to the way of direct collection of signals in extracts, the tedious and complicated multiple magnetic separation and signal amplification procedures in conventional methods were avoided, thus the procedures were significantly simplified, and the reduction of the operation time of 30 min for sample pretreatment and 40 min for detection part was achieved. The biosensor might be promising in the rapid, in-field and sensitive screening of multiple veterinary drugs to ensure agriculture and food safety.








Similar content being viewed by others
References
Nisha AR. Antibiotic residues—a global health hazard. Veterinary. 2008;1(12):375–377.
Demain AL, Sanchez S. Microbial drug discovery: 80 years of progress. J Antibiot (Tokyo). 2009;62(1):5–16.
Fiaz A, Zhu D, Sun J. Environmental fate of tetracycline antibiotics: degradation pathway mechanisms, challenges, and perspectives. Environ Sci Eur. 2021;33:64.
Kumar RR, Lee JT, Cho JY. Fate, occurrence, and toxicity of veterinary antibiotics in environment. J Korean Soc Appl Biol Chem. 2012;55(6):701–9.
Liu X, Steele JC, Meng XZ. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ Pollut. 2017;223:161–9.
Zheng Q, Zhang R, Wang Y, Pan X, Tang J, Zhang G. Occurrence and distribution of antibiotics in the Beibu Gulf, China: impacts of river discharge and aquaculture activities. Mar Environ Res. 2012;78:26–33.
Cowieson AJ, Kluenter AM. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim Feed Sci Technol. 2019;250:81–92.
Kovalakova P, Cizmas L, McDonald TJ, Marsalek B, Feng M, Sharma VK. Occurrence and toxicity of antibiotics in the aquatic environment: a review. Chemosphere. 2020;251:126351.
Muhammad J, Khan S, Su JQ, Hesham AE-L, Ditta A, Nawab J, Ali A. Antibiotics in poultry manure and their associated health issues: a systematic review. J Soils Sediments. 2019;20(1):486–97.
Ben Y, Fu C, Hu M, Liu L, Wong MH, Zheng C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: a review. Environ Res. 2019;169:483–93.
Hendriksen RS, Munk P, Njage P, van Bunnik B, McNally L, Lukjancenko O, Roder T, Nieuwenhuijse D, Pedersen SK, Kjeldgaard J, Kaas RS, Clausen P, Vogt JK, Leekitcharoenphon P, van de Schans MGM, Zuidema T, de Roda Husman AM, Rasmussen S, Petersen B, Global Sewage Surveillance project c, Amid C, Cochrane G, Sicheritz-Ponten T, Schmitt H, Alvarez JRM, Aidara-Kane A, Pamp SJ, Lund O, Hald T, Woolhouse M, Koopmans MP, Vigre H, Petersen TN, Aarestrup FM. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun. 2019;10(1):1124.
Lorenzo P, Adriana A, Jessica S, Carles B, Marinella F, Marta L, Luis BJ, Pierre S. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere. 2018;206:70–82.
Subirats J, Di Cesare A, Varela Della Giustina S, Fiorentino A, Eckert EM, Rodriguez-Mozaz S, Borrego CM, Corno G. High-quality treated wastewater causes remarkable changes in natural microbial communities and intI1 gene abundance. Water Res. 2019;167:114895.
Zarei-Baygi A, Harb M, Wang P, Stadler LB, Smith AL. Evaluating antibiotic resistance gene correlations with antibiotic exposure conditions in anaerobic membrane bioreactors. Environ Sci Technol. 2019;53(7):3599–609.
Blanchaert B, Poderós Jorge E, Jankovics P, Adams E, Van Schepdael A. Assay of Kanamycin A by HPLC with Direct UV Detection. Chromatographia. 2013;76(21–22):1505–12.
Posyniak A, Zmudzki J, Niedzielska J. Evaluation of sample preparation for control of chloramphenicol residues in porcine tissues by enzyme-linked immunosorbent assay and liquid chromatography. Anal Chim Acta. 2003;483(1–2):307–11.
Gülfen M, Canbaz Y, Özdemir A. Simultaneous Determination of amoxicillin, lansoprazole, and levofloxacin in pharmaceuticals by HPLC with UV–Vis detector. J Anal Testing. 2020;4(1):45–53.
Holstege DM, Puschner B, Whitehead G, Galey FD. Screening and mass spectral confirmation of β-Lactam antibiotic residues in Milk using LC-MS/MS. J Agric Food Chem 2002;50:406–411.
Xie X, Wang B, Pang M, Zhao X, Xie K, Zhang Y, Wang Y, Guo Y, Liu C, Bu X, Wang R, Shi H, Zhang G, Zhang T, Dai G, Wang J. Quantitative analysis of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in eggs via liquid chromatography-electrospray ionization tandem mass spectrometry. Food Chem. 2018;269:542–8.
Liu T, Xie J, Zhao J, Song G, Hu Y. Magnetic chitosan nanocomposite used as cleanup material to detect chloramphenicol in milk by GC-MS. Food Anal Methods. 2013;7(4):814–9.
Wang W, Wang R, Liao M, Kidd MT, Li Y. Rapid detection of enrofloxacin using a localized surface plasmon resonance sensor based on polydopamine molecular imprinted recognition polymer. J Food Meas Charact. 2021;15:3376–86.
Adrian J, Pinacho DG, Granier B, Diserens JM, Sanchez-Baeza F, Marco MP. A multianalyte ELISA for immunochemical screening of sulfonamide, fluoroquinolone and ss-lactam antibiotics in milk samples using class-selective bioreceptors. Anal Bioanal Chem. 2008;391(5):1703–12.
Chen Y, Chen Q, Han M, Liu J, Zhao P, He L, Zhang Y, Niu Y, Yang W, Zhang L. Near-infrared fluorescence-based multiplex lateral flow immunoassay for the simultaneous detection of four antibiotic residue families in milk. Biosens Bioelectron. 2016;79:430–4.
Hendrickson OD, Byzova NA, Zvereva EA, Zherdev AV, Dzantiev BB. Sensitive lateral flow immunoassay of an antibiotic neomycin in foodstuffs. J Food Sci Technol. 2021;58(1):292–301.
Jiang W, Wang Z, Beier RC, Jiang H, Wu Y, Shen J. Simultaneous determination of 13 fluoroquinolone and 22 sulfonamide residues in milk by a dual-colorimetric enzyme-linked immunosorbent assay. Anal Chem. 2013;85(4):1995–9.
Hao L, Chen J, Chen X, Ma T, Cai X, Duan H, Leng Y, Huang X, Xiong Y. A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice. Food Chem. 2021;336:127710.
Huang Z, Xiong Z, Chen Y, Hu S, Lai W. Sensitive and matrix-tolerant lateral flow immunoassay based on fluorescent magnetic nanobeads for the detection of clenbuterol in swine urine. J Agric Food Chem. 2019;67(10):3028–36.
Ren M, Xu H, Huang X, Kuang M, Xiong Y, Xu H, Xu Y, Chen H, Wang A. Immunochromatographic assay for ultrasensitive detection of aflatoxin B1 in maize by highly luminescent quantum dot beads. ACS Appl Mater Interfaces. 2014;6(16):14215–22.
Li F, Guo Y, Wang X, Sun X. Multiplexed aptasensor based on metal ions labels for simultaneous detection of multiple antibiotic residues in milk. Biosens Bioelectron. 2018;115:7–13.
Liu S, Ding J, Qin W. Dual-analyte chronopotentiometric aptasensing platform based on a G-Quadruplex/Hemin DNAzyme and logic gate operations. Anal Chem. 2019;91(4):3170–6.
Hong CY, Zhang XX, Dai CY, Wu CY, Huang ZY. Highly sensitive detection of multiple antibiotics based on DNA tetrahedron nanostructure-functionalized magnetic beads. Anal Chim Acta. 2020;1120:50–8.
Youn H, Lee K, Her J, Jeon J, Mok J, So JI, Shin S, Ban C. Aptasensor for multiplex detection of antibiotics based on FRET strategy combined with aptamer/graphene oxide complex. Sci Rep. 2019;9(1):7659.
Fang B, Xu S, Huang Z, Wang S, Chen W, Yuan M, Hu S, Peng J, Lai W. Glucose oxidase-induced colorimetric immunoassay for qualitative detection of danofloxacin based on iron (II) chelation reaction with phenanthroline. Food Chem. 2020;328:127099.
Zhao M, Li X, Zhang Y, Wang Y, Wang B, Zheng L, Zhang D, Zhuang S. Rapid quantitative detection of chloramphenicol in milk by microfluidic immunoassay. Food Chem. 2021;339:127857.
Xu T, Jia X, Chen X, Ma Z. Simultaneous electrochemical detection of multiple tumor markers using metal ions tagged immunocolloidal gold. Biosens Bioelectron. 2014;56:174–9.
Zhu YL, Lian YM, Wang JK, Chen ZP, Yu RQ. Highly Sensitive and specific mass spectrometric platform for mirna detection based on the multiple-metal-nanoparticle tagging strategy. Anal Chem. 2021;93(14):5839–48.
Lisi F, Falcaro P, Buso D, Hill AJ, Barr JA, Crameri G, Nguyen TL, Wang LF, Mulvaney P. Rapid detection of hendra virus using magnetic particles and quantum dots. Adv Health Mater. 2012;1(5):631–4.
Becheva ZR, Ivanov YL, Godjevargova TI, Tchorbanov AI. Simultaneous determination of ochratoxin A and enterotoxin A in milk by magnetic nanoparticles based fluorescent immunoassay. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2021;1–19.
Chang J, Wang X, Wang J, Li H, Li F. Nucleic acid-functionalized metal-organic framework-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal Chem. 2019;91(5):3604–10.
Cheng Z, Choi N, Wang R, Lee S, Moon KC, Yoon SY, Chen L, Choo J. Simultaneous detection of dual prostate specific antigens using surface-enhanced Raman Scattering-Based Immunoassay for Accurate Diagnosis of Prostate Cancer. ACS Nano. 2017;11(5):4926–33.
Shimron S, Wang F, Orbach R, Willner I. Amplified detection of DNA through the enzyme-free autonomous assembly of hemin/G-quadruplex DNAzyme nanowires. Anal Chem. 2012;84(2):1042–8.
Xia X, Wang Y, Yang H, Dong Y, Zhang K, Lu Y, Deng R, He Q. Enzyme-free amplified and ultrafast detection of aflatoxin B1 using dual-terminal proximity aptamer probes. Food Chem. 2019;283:32–8.
Li S, Wen W, Guo J, Wang S, Wang J. Development of non-enzymatic and photothermal immuno-sensing assay for detecting the enrofloxacin in animal derived food by utilizing black phosphorus-platinum two-dimensional nanomaterials. Food Chem. 2021;357:129766.
Sha J, Lin H, Timira V, Sui J. The construction and application of aptamer to simultaneous identification of enrofloxacin and ciprofloxacin residues in fish. Food Anal Methods. 2021;14(5):957–67.
Zhang Y, Duan B, Bao Q, Yang T, Wei T, Wang J, Mao C, Zhang C, Yang M. Aptamer-modified sensitive nanobiosensors for the specific detection of antibiotics. J Mater Chem B. 2020;8(37):8607–13.
Shen Y, He Y, Fu Y, Wang J, Zhang J, Liao M, Li Y. A Nanomaterial-based biosensor for the quantitative detection of enrofloxacin residues in raw chicken. T ASABE. 2020;63(6):1869–78.
Acknowledgements
This research was funded by the Walmart Foundation (0402-70013-21-0000) and supported by the Walmart Food Safety Collaboration Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Peng, YP., He, YW., Shen, YF. et al. Fluorescence Nanobiosensor for Simultaneous Detection of Multiple Veterinary Drugs in Chicken Samples. J. Anal. Test. 6, 77–88 (2022). https://doi.org/10.1007/s41664-021-00199-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-021-00199-4