Abstract
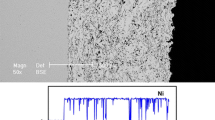
The effects of introducing small amounts of phosphorus (P) into α-brass on its corrosion behavior in Azrou soil were examined. Three α-brasses (denoted EM1, EM2, and EM3) with different P contents were studied in this work. These brasses were investigated using Electrochemical Impedance Spectroscopy (EIS), potentiodynamic polarization (PDP) plots and Scanning Electron Microscopy (SEM), whereas the chemical composition was determined by energy-dispersive X-ray spectroscopy (EDS). The experimental results showed that the most resistant of the three α-brasses to corrosion in this soil was EM3. Moreover, the presence of P in the α-brasses decreased their corrosion rates and increased their corrosion resistance, i.e., the polarization resistance of the brasses to corrosion increased with the P content of the brass. The maximum polarization resistance, Rp = 851 kΩ cm2, occurred with a P content of 0.137%. The observed changes in surface morphology with increasing P content and the corrosion products deposited on the brass surface were examined by X-ray diffraction. EM3 coupons in the studied soil only showed base metal peaks, demonstrating that EM3 is highly resistant to corrosion.














Similar content being viewed by others
References
Abedini M, Ghasemi HM (2014) Synergistic erosion–corrosion behavior of Al–brass alloy at various impingement angles. Wear 319(1–2):49–55
Alfantazi AM, Ahmed TM, Tromans D (2009) Corrosion behavior of copper alloys in chloride media. Mater Des 30(7):2425–2430
Amar H, Benzakour J, Derja A, Villemin D, Moreau B, Braisaz TJ (2006) Piperidin-1-yl-phosphonic acid and (4-phosphono-piperazin-1-yl) phosphonic acid: a new class of iron corrosion inhibitors in sodium chloride 3% media. Appl Surf Sci 252:6162–6172
Badawy WA, El-Egamy SS, El-Azab AS (1995) The electrochemical behaviour of leaded brass in neutral CL− and SO −4 media. Corrosion Sci 37:1969
Badawy WA, El-Rabiei MM, Nady H (2014) Synergistic effects of alloying elements in Cu-ternary alloys in chloride solutions. Electrochim Acta 120:39–45
Barbalat M, Lanarde L, Caron D, Meyer M, Vittonato J, Castillon F, Fontaine S, Refait P (2012) Electrochemical study of the corrosion rate of carbon steel in soil: evolution with time and determination of residual corrosion rates under cathodic protection. Corros Sci 55:246–253
Barbalat M, Caron D, Lanarde L, Meyer M, Fontaine S, Castillon F, Vittonato J, Refait P (2013) Estimation of residual corrosion rates of steel under cathodic protection in soils via voltammetry. Corros Sci 73:222–229
Barcia OE, D’Elia E, Frateur I, Mattos OR, Pébère N, Tribollet B (2002) Application of the impedance model of de Levie for the characterization of porous electrodes. Electrochim Acta 47:2109–2116
Bin H, Pengju H, Chunhui L, Xiaohong B (2015) Effect of soil particle size on the corrosion behavior of natural gas pipeline. Eng Fail Anal 58:19–30
Bouckamp A (1993) Users manual: Equivalent Circuit ver. 4:51. University of Twente, Enschede
Bowers JE, Oseland PWR, Davies GC (1978) Development of a hot-stamping brass resistant to dezincification. Br Corros J 13:177
Bradford SA (2003) Practical handbook of corrosion control in soils. CASTI, Edmonton, p 60
Caleyo F, Velázquez JC, Valor A, Hallen JM (2009) Probability distribution of pitting corrosion depth and rate in underground pipelines: a Monte Carlo study. Corros Sci 51:1925–1934
Chen B, Liang C, Fu D, Ren D (2005) Corrosion behavior of Cu and the Cu–Zn–Al shape memory alloy in simulated uterine fluid. Contraception 72:221–224
Corcoran P, Jarvis MG, Mackney D, Stevens KW (1977) Soil corrosiveness in South Oxfordshire. J Soil Sci 28:473–484
Dang DN, Lanarde L, Jeannin M, Sabot R, Refait P (2015) Influence of soil moisture on the residual corrosion rates of buried carbon steel structures under cathodic protection. Electrochim Acta 176:1410–1419
Davies DD (1993) A note on the dezincification of brass and the inhibiting effect of elemental additions. Copper Development Association Inc., New York, pp 1–9
Du XS, Su YJ, Li JX, Qiao LJ, Chu WY (2012) Inhibitive effects and mechanism of phosphates on the stress corrosion cracking of brass in ammonia solutions. Corros Sci 60:69–75
El Fadil EH, Azaroual MA, Touhami ME, Nassali H, Benqlilou H, Berrami K, Ouaki B (2018) A comparative study of the behavior of several types of complex brass marketed in the Moroccan market during the process of corrosion in synthetic water. Moroccan J Chem 6(3):6–3
Elhousni L, Galai M, ElKamraoui FZ et al (2017) Corrosion and scale studies of copper used in Moroccan industrial cooling water systems. Euro-Mediterr J Environ Integr 2:12. https://doi.org/10.1007/s41207-017-0024-y
Fiaud C, Bensarsa S, des Aulnois ID, des Aulnois DM (1987) Inhibiting properties of phosphines against zinc corrosion in acidic media. Br Corros J 22:109
Folquer ME, Ribotta SB, Real SG, Gassa LM (2002) Inhibiting properties of phosphines against zinc corrosion in acidic media. Br Corros J 58(3):240
Galai M, Ouassir J, Ebn-Touhami M, Nassali H, Benqlilou H, Belhaj T, Berrami K, Mansouri I, Oauki B (2017) α-Brass and (α+β) brass degradation processes in Azrou soil medium used in plumbing devices. J Bio- Tribo-Corros 3:30
Galai M, Benqlilou H, Touhami ME, Belhaj T, Berrami K, El Kafssaoui H (2018) Comparative analysis for the corrosion susceptibility of copper alloys in sandy soil. Environ Eng Res 23(2):164–174
Galai M, Choucri J, Hassani Y, Benqlilou H, Mansouri I, Ouaki B, Ebn-Touhami M, Monticelli C, Zucchi F (2019) Moisture content and chloride ion effect on the corrosion behavior of fitting brass (gate valves) used as a connection of PVC’s conduits in aggressive sandy soil. Chem Data Collect 19:100171
Gopiraman M, Selvakumaran N, Kesavan D (2012) Adsorption and corrosion inhibition behaviour of N-(phenylcarbamothioyl) benzamide on mild steel in acidic medium. Prog Org Coat 73(1):104–111
Guo XJ, Gao KW, Qiao LJ, Chu WY (2002) The correspondence between susceptibility to SCC of brass and corrosion-induced tensile stress with various pH values. Corrosion Sci 44:2367
Gupta SK, Gupta BK (1979) The critical soil moisture content in the underground corrosion of mild steel. Corros Sci 19:171–178
ISO (1991) Soil Quali@—terminology—soil data structuration. AFNOR, Paris
Jiang J, Wang J, Wang WW, Zhang W (2009) Modeling influence of gas/liquid/solid three-phase boundary zone on cathodic process of soil corrosion. Electrochim Acta 54:3623–3629
Jorcin JB (2007) Spectroscopie d'impédance électrochimique locale: caractérisation de la de lamination des peintures et de la corrosion des alliages Al-Cu. Thesis. INPT, Toulouse
Karpagavalli R, Balasubramaniam AER (2007) Influence of arsenic, antimony and phosphorous on the microstructure and corrosion behavior of brasses. J Mater Sci 42:5954–5958
Kear G, Barker BD, Stokes KR, Walsh FC (2004a) Flow influenced electrochemical corrosion of nickel aluminium bronze—part II. Anodic polarization and derivation of the mixed potential. J Appl Electrochem 34:1241–1248
Kear G, Barker BD, Stokes KR, Walsh FC (2004b) Flow influenced electrochemical corrosion of nickel aluminium bronze—part I. Cathodic polarization. J Appl Electrochem 34:1235–1240
Lehockey EM, Brennenstuhl AM, Thompson I (2004) Influence of arsenic, antimony and phosphorous on the microstructure and corrosion behavior of brasses. Corros Sci 46:2383
Liao X, Cao F, Zheng L, Liu W, Chen A, Zhang J, Cao C (2011) Corrosion behaviour of copper under chloride-containing thin electrolyte layer. Corros Sci 53(10):3289–3298
Metikos M, Hukovic SI, Grubac Z, Babic R (2010) Complexities of corrosion behavior of copper–nickel alloys under liquid impingement conditions in saline water. Electrochim Acta 55:3123–3129
Moore TJ, Halmark CT (1987) Soil properties influencing corrosion of steel in Texas soils. Soil Sci Soc Am J 51:1250–1256
Nady H, El-Rabiei MM, Samy M, Badawy WA (2020) The influence of alloying elements (Al, Ni and Zn) on the corrosion resistance of some Cu-ternary alloys in Na2SO4 solutions. J Bio Tribocorros 6(1):22
Newman RC (2008) Stress corrosion cracking of noble metals and their alloys in solutions containing cations of the noble metal: review of observations relevant to competing models of SCC. Corros Sci 50(7):1807–1810
Procaccini R, Ceré S, Vázquez M (2009) Oxygen reduction on Cu–Zn alloys. J Appl Electrochem 39:177–184
Revie RW, Uhlig HH (2008) Corrosion and corrosion control: an introduction to corrosion science and engineering. Wiley, Hoboken
Robinson WC (1993) Testing soil for corrosiveness. Mater Perform 32:56–58
Sabbaghzadeh B, Parvizi R, Davoodi A, Moayed MH (2014) Corrosion evaluation of multi-pass welded nickel–aluminum bronze alloy in 3.5% sodium chloride solution: a restorative application of gas tungsten arc welding process. Mater Des 58:346–356
Sarver E, Zhang Y, Edwards M (2010a) Review of brass dezincification corrosion in potable water systems. Corros Rev 28(3–4):155–196
Sarver E, Zang Y, Edwards M (2010b) Review of brass dezincification corrosion in potable water systems. Corros Rev 28:155
Sayed SM, Ashour EA, Youssef GI (2003) Effect of sulfide ions on the corrosion behaviour of Al–brass and Cu10Ni alloys in salt water. Mater Chem Phys 78:825
Selvaraj S, Ponmariappan S, Natesan M, Palaniswamy N (2003) Dezincification of brass and its control—an overview. Corros Rev 21(1):41–74
Seuss F, Gaag N, Virtanen S (2017) Corrosion mechanism of CuZn21Si3P in aggressive tap water. Mater Corros 68:42–49. https://doi.org/10.1002/maco.201609018
Shim J, Kim J (2004) Copper corrosion in potable water distribution systems: influence of copper products on the corrosion behavior. Mater Lett 58:2002
Stern M, Geary AL (1957) Discussion of electrochemical polarization, 1. A theoretical analysis of the shape of polarization curves. J Electrochem Soc 104:56–63
Trabanelli G, Monticelli C, Grassi V, Frignani A (2005) Electrochemical study on inhibitors of rebar corrosion in carbonated concrete. Cem Concr Res 35:1804–1813
Valcarce MB, Sanchez SR, Vazquez M (2005) Corrosion inhibitors: design, performance, and computer simulations. Corros Sci 47:795
Valor A, Caleyo F, Hallen JM, Velázquez JC (2013) Reliability assessment of buried pipelines based on different corrosion rate models. Corros Sci 66:78–87
Vazquez VM, Sánchez SR, Calvo EJ, Schiffrin DJ (1994) The electrochemical reduction of oxygen on polycrystalline copper in borax buffer. J Electroanal Chem 374:189–197
Yohai L, Schreiner WH, Vazquez M, Valcarce ME (2011) Surface characterization of copper, zinc and brass in contact with tap water inhibited with phosphate ions. Appl Surf Sci 257:10089–10095
You SJ, Choi YS, Kim JG, Oh HJ, Chi CS (2003) Flow behavior of lead-free machinable brass during hot compression deformation. Mater Sci Eng A 345:207
Zucchi F, Trabanelli G, Fonsati M, Giusti A (1998) Stress corrosion cracking of 13% Cr martensitic steels in sodium chloride solutions in the presence of thiosulphate. Mater Corros 49:864
Funding
The authors gratefully acknowledge the National Office of Electricity and Drinking Water (ONEE), Rabat, Morocco, for financially assisting with and facilitating our study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Communicated by Abdelwaheb Aydi, Associate Editor.
Rights and permissions
About this article
Cite this article
Galai, M., Benqlilou, H., EbnTouhami, M. et al. Effect of phosphorus content of α-brass on its corrosion resistance in aggressive soil: experimental and characterization studies. Euro-Mediterr J Environ Integr 6, 41 (2021). https://doi.org/10.1007/s41207-021-00244-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-021-00244-9