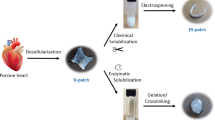
Abstract
Effective cardiac tissue regeneration necessitates scaffolds that mimic the native extracellular matrix and possess desirable properties, such as electrical conductivity and biocompatibility. The choice of an appropriate fabrication method is paramount in achieving reproducibility, scalability, and rapid production of cardiac tissue patches. Electrospinning, a versatile and widely utilized technique, offers precise control over fiber diameter, pore size, and alignment, rendering it an ideal method for creating intricate cardiac scaffolds. In light of the limitations of existing therapies and the need for innovative approaches, this research aims to explore the development of novel patches for cardiac tissue regeneration. By investigating the integration of MXenes into electrospun polycaprolactone (PCL) membranes, we aim to harness the unique properties of MXenes to create conductive, biocompatible, and mechanically robust scaffolds that promote cell adhesion, proliferation, and functional maturation. The application of oxygen plasma treatment enhances the infiltration of MXene into the PCL electrospun membrane, significantly reducing the surface contact angle and promoting cell adhesion. Regardless of the number of MXene deposition repetitions, all variants demonstrated strong biocompatibility and supported the formation of cell symplasts after fibroblast seeding. The remarkable electrical conductivity of PCL-MXene membranes, coupled with the positive biological outcomes presented in this study, has the potential to drive significant advancements in the field of cardiac tissue engineering. This research offers fresh insights and approaches to tackle the challenges associated with myocardial repair and regeneration.












Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study. The raw data are available on request.
References
Mortality and global health estimates (2022) [Online]. Available: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019.
European Cardiovascular Disease Statistics (2017) edition
Baei P, Hosseini M, Baharvand H, Pahlavan S (2020) Electrically conductive materials for in vitro cardiac microtissue engineering. J Biomed Mater Res Part A 108(5):1203–1213. https://doi.org/10.1002/JBM.A.36894
Alegret N, Dominguez-Alfaro A, Mecerreyes D (2019) 3D scaffolds based on conductive polymers for biomedical applications. Biomacromol 20(1):73–89. https://doi.org/10.1021/ACS.BIOMAC.8B01382/ASSET/IMAGES/LARGE/BM-2018-013823_0013.JPEG
Yanamandala M et al (2017) Overcoming the roadblocks to cardiac cell therapy using tissue engineering. J Am Coll Cardiol 70(6):766–775. https://doi.org/10.1016/J.JACC.2017.06.012
Weinberger F, Mannhardt I, Eschenhagen T (2017) Engineering cardiac muscle tissue: a maturating field of research. Circ Res 120(9):1487–1500. https://doi.org/10.1161/CIRCRESAHA.117.310738
Scott L, Jurewicz I, Jeevaratnam K, Lewis R (2021) Carbon nanotube-based scaffolds for cardiac tissue engineering—Systematic review and narrative synthesis. Bioengineering. https://doi.org/10.3390/BIOENGINEERING8060080
You JO, Rafat M, Ye GJC, Auguste DT (2011) Nanoengineering the heart: conductive scaffolds enhance connexin 43 expression. Nano Lett 11(9):3643–3648. https://doi.org/10.1021/NL201514A/SUPPL_FILE/NL201514A_SI_002.AVI
Orza A et al (2011) Electrically conductive gold-coated collagen nanofibers for placental-derived mesenchymal stem cells enhanced differentiation and proliferation. ACS Nano 5(6):4490–4503. https://doi.org/10.1021/NN1035312/SUPPL_FILE/NN1035312_SI_001.PDF
Ravichandran R, Sridhar R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S (2014) Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration. Macromol Biosci 14(4):515–525. https://doi.org/10.1002/MABI.201300407
Baei P, Jalili-Firoozinezhad S, Rajabi-Zeleti S, Tafazzoli-Shadpour M, Baharvand H, Aghdami N (2016) Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater Sci Eng C Mater Biol Appl 63:131–141. https://doi.org/10.1016/J.MSEC.2016.02.056
Sherrell PC et al (2017) Rational design of a conductive collagen heart patch. Macromol Biosci 17(7):1600446. https://doi.org/10.1002/MABI.201600446
Wu Y, Wang L, Guo B, Ma PX (2017) Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3d cardiac anisotropy. ACS Nano 11(6):5646–5659. https://doi.org/10.1021/ACSNANO.7B01062/SUPPL_FILE/NN7B01062_SI_008.AVI
Izadifar M, Chapman D, Babyn P, Chen X, Kelly ME (2018) UV-assisted 3d bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods 24(2):74–88. https://doi.org/10.1089/TEN.TEC.2017.0346
Ahadian S et al (2017) Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater 52:81–91. https://doi.org/10.1016/J.ACTBIO.2016.12.009
Hitscherich P et al (2018) Electroactive graphene composite scaffolds for cardiac tissue engineering. J Biomed Mater Res A 106(11):2923–2933. https://doi.org/10.1002/JBM.A.36481
Bao R, Tan B, Liang S, Zhang N, Wang W, Liu W (2017) A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterial 122:63–71. https://doi.org/10.1016/J.BIOMATERIALS.2017.01.012
Smith AST et al (2017) Micro- and nano-patterned conductive graphene–PEG hybrid scaffolds for cardiac tissue engineering. Chem Commun 53(53):7412–7415. https://doi.org/10.1039/C7CC01988B
Liu H et al (2023) An electroconductive hydrogel scaffold with injectability and biodegradability to manipulate neural stem cells for enhancing spinal cord injury repair. Biomacromolecule 24(1):86–97. https://doi.org/10.1021/acs.biomac.2c00920
Furlani F et al (2023) Electroconductive scaffolds based on gelatin and PEDOT:PSS for cardiac regeneration. Int J Biol Macromol 224:266–280. https://doi.org/10.1016/j.ijbiomac.2022.10.122
Srinivasan SY et al (2023) Conductive bacterial nanocellulose-polypyrrole patches promote cardiomyocyte differentiation. ACS Appl Bio Mater. https://doi.org/10.1021/acsabm.3c00303
Ghosh S, Dhiman M, Gupta S, Roy P, Lahiri D (2023) Electro-conductive chitosan/graphene bio-nanocomposite scaffold for tissue engineering of the central nervous system. Biomaterial. https://doi.org/10.1016/j.bioadv.2023.213596
Nekounam H et al (2021) Electroconductive scaffolds for tissue regeneration: Current opportunities, pitfalls, and potential solutions. Mater Res Bull. https://doi.org/10.1016/j.materresbull.2020.111083
Gogotsi Y, Anasori B (2019) The Rise of MXenes. ACS Nano 13(8):8491–8494. https://doi.org/10.1021/acsnano.9b06394
Gokce C, Gurcan C, Delogu LG, Yilmazer A (2022) 2D materials for cardiac tissue repair and regeneration. Front Cardiovasc Med. https://doi.org/10.3389/FCVM.2022.802551
Gazzi A et al (2019) Photodynamic therapy based on graphene and MXene in cancer theranostics. Front Bioeng Biotechnol. https://doi.org/10.3389/FBIOE.2019.00295/BIBTEX
Kyrylenko S et al (2022) MXene-assisted ablation of cells with a pulsed near-infrared laser. ACS Appl Mater Interfaces 14(25):28683–28696. https://doi.org/10.1021/ACSAMI.2C08678/ASSET/IMAGES/LARGE/AM2C08678_0011.JPEG
Fusco L et al (2023) V4C3 MXene immune profiling and modulation of t cell-dendritic cell function and interaction. Small Methods. https://doi.org/10.1002/smtd.202300197
Fusco L et al (2022) Immune profiling and multiplexed label-free detection of 2d mxenes by mass cytometry and high-dimensional imaging. Adv Mater. https://doi.org/10.1002/adma.202205154
Peng G, Keshavan S, Delogu L, Shin Y, Casiraghi C, Fadeel B (2022) Two-dimensional transition metal dichalcogenides trigger trained immunity in human macrophages through epigenetic and metabolic pathways. Small 18:20. https://doi.org/10.1002/smll.202107816
Driscoll N et al (2021) MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci Transl Med. https://doi.org/10.1126/SCITRANSLMED.ABF8629/SUPPL_FILE/SCITRANSLMED.ABF8629_DATA_FILE_S1.ZIP
Wang Y et al (2021) Ti3C2TxMXene flakes for optical control of neuronal electrical activity. ACS Nano 15(9):14662–14671. https://doi.org/10.1021/ACSNANO.1C04431/SUPPL_FILE/NN1C04431_SI_004.PDF
Li Y et al (2023) Toward Smart Sensing by MXene. Small. https://doi.org/10.1002/smll.202206126
Lai QT, Zhao XH, Sun QJ, Tang Z, Tang XG, Roy VAL (2023) Emerging MXene-based flexible tactile sensors for health monitoring and haptic perception. Small. https://doi.org/10.1002/smll.202300283
Manisekaran R, Chettiar ADR, Kandasamy G, GarciaContreras R, AcostaTorres LS (2023) State of the art: MXene structures in nano oncology. Biomater Adv 147:213354. https://doi.org/10.1016/j.bioadv.2023.213354
Solangi NH, Mazari SA, Mubarak NM, Karri RR, Rajamohan N, Vo DVN (2023) Recent trends in Mxene based material for biomedical applications. Environ Res 222:115337. https://doi.org/10.1016/j.envres.2023.115337
Schmitt PR, Dwyer KD, Coulombe KLK (2022) Current applications of polycaprolactone as a scaffold material for heart regeneration. ACS Appl Bio Mater 5(6):2461–2480. https://doi.org/10.1021/acsabm.2c00174
Park D, Lee SJ, Choi DK, Park JW (2023) Therapeutic agent-loaded fibrous scaffolds for biomedical applications. Pharmaceutics. https://doi.org/10.3390/pharmaceutics15051522
Kołtunowicz TN et al (2021) Investigation of AC electrical properties of mxene-pcl nanocomposites for application in small and medium power generation. Energies 14(21):7123. https://doi.org/10.3390/en14217123
Diedkova K et al. (2022) The Multistep Process of Coating PCL Membranes with MXene Solution pp. NRA17–1-NRA17–4: https://doi.org/10.1109/NAP55339.2022.9934231
Kyrylenko S et al. (2020) Bio-Functionalization of Electrospun Polymeric Nanofibers by Ti3CTx MXene Proc 2020 IEEE 10th Int. Conf. Nanomaterials Appl. Prop. N. 2020: https://doi.org/10.1109/NAP51477.2020.9309612
Diedkova K et al (2022) Polycaprolactone-MXene nanofibrous scaffolds for tissue engineering. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.2c22780
Seddighian A, Ganji F, Baghaban-Eslaminejad M, Bagheri F (2021) Electrospun PCL scaffold modified with chitosan nanoparticles for enhanced bone regeneration. Prog Biomater 10(1):65–76. https://doi.org/10.1007/s40204-021-00153-8
Zaret BL, Beller GA (2010) Clinical nuclear cardiology: state of the art and future directions. Clin Nucl Cardiol State Art Futur Dir 12:1–878. https://doi.org/10.1016/C2009-0-53360-0
Tayebi T et al (2021) Biofabrication of chitosan/chitosan nanoparticles/polycaprolactone transparent membrane for corneal endothelial tissue engineering. Sci Rep. https://doi.org/10.1038/S41598-021-86340-W
Coleman MM, Zarian J (1979) Fourier-transform infrared studies of polymer blends. II. Poly(ϵ-caprolactone)–poly(vinyl chloride) system. J Polym Sci Polym Phys Ed 17(5):837–850. https://doi.org/10.1002/POL.1979.180170509
Hu T, Hu M, Gao B, Li W, Wang X (2018) Screening surface structure of mxenes by high-throughput computation and vibrational spectroscopic confirmation. J Phys Chem C 122(32):18501–18509. https://doi.org/10.1021/ACS.JPCC.8B04427/SUPPL_FILE/JP8B04427_SI_001.PDF
Korniienko V et al (2021) Functional and biological characterization of chitosan electrospun nanofibrous membrane nucleated with silver nanoparticles. Appl Nanosci. https://doi.org/10.1007/s13204-021-01808-5
Fan H, Guo Z (2020) Bioinspired surfaces with wettability: biomolecule adhesion behaviors. Biomater Sci 8(6):1502–1535. https://doi.org/10.1039/C9BM01729A
Lin WC, Razali NAM (2019) Temporary wettability tuning of PCL/PDMS micro pattern using the plasma treatments. Mater 12:644. https://doi.org/10.3390/MA12040644
Basara G et al (2022) Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater 139:179–189. https://doi.org/10.1016/J.ACTBIO.2020.12.033
Liang Y, Mitriashkin A, Lim TT, Goh JCH (2021) Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. Biomaterial 276:121008. https://doi.org/10.1016/J.BIOMATERIALS.2021.121008
Sundaram A, Francis BM, Dhanabalan SC, Ponraj JS (2021) Transition metal carbide—MXene. Handb carbon-based nanomater. Elsevier, pp 671–709
Sang M et al (2022) Advanced MXene/shear stiffening composite-based sensor with high-performance electromagnetic interference shielding and anti-impacting Bi-protection properties for smart wearable device. Chem Eng J. https://doi.org/10.1016/J.CEJ.2022.135869
Gil-Cabrerizo P, Scacchetti I, Garbayo E, Blanco-Prieto MJ (2023) Cardiac tissue engineering for myocardial infarction treatment. Eur J Pharm Sci. https://doi.org/10.1016/j.ejps.2023.106439
Yu Z et al (2023) 3D conductive scaffolds of MXene@PCL with high conductivity and small line width fabricated by electric-field-driven jet 3D printing and electrostatic self-assembly. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2023.105704
Shokrollahi P, Omidi Y, Cubeddu LX, Omidian H (2023) Conductive polymers for cardiac tissue engineering and regeneration. J Biomed Mater Res Part B Appl Biomater. https://doi.org/10.1002/jbm.b.35293
Akbarzadeh A, Sobhani S, SoltaniKhaboushan A, Kajbafzadeh AM (2023) Wholeheart tissue engineering and cardiac patches: challenges and promises. Bioengineering. https://doi.org/10.3390/bioengineering10010106
Wang B et al (2023) Functional acellular matrix for tissue repair. Mater. Today Bio 18:100530. https://doi.org/10.1016/j.mtbio.2022.100530
Salaris V, López D, Kenny JM, Peponi L (2023) Hydrolytic degradation and bioactivity of electrospun PCL-MG-NPS fibrous mats. Molecules. https://doi.org/10.3390/molecules28031001
Dias JR, Sousa A, Augusto A, Bártolo PJ, Granja PL (2022) Electrospun Polycaprolactone (PCL) degradation: an in vitro and in vivo study. Polymers (Basel). https://doi.org/10.3390/polym14163397
Ye G et al (2020) Mussel-inspired conductive Ti2C-cryogel promotes functional maturation of cardiomyocytes and enhances repair of myocardial infarction. Theranostics 10(5):2047–2066. https://doi.org/10.7150/THNO.38876
Rafieerad A et al (2019) Quantum dots: application of Ti3C2 MXene quantum dots for immunomodulation and regenerative medicine (Adv Healthcare Mater 16/2019). Adv Healthc Mater 8(16):1970067. https://doi.org/10.1002/ADHM.201970067
Rafieerad A et al (2021) Fabrication of smart tantalum carbide Mxene quantum dots with intrinsic immunomodulatory properties for treatment of allograft vasculopathy. Adv Funct Mater 31(46):2170341. https://doi.org/10.1002/ADFM.202170341
Asaro GA et al (2023) MXene functionalized collagen biomaterials for cardiac tissue engineering driving iPSC-derived cardiomyocyte maturation. Npj 2D Mater Appl 71(1):1–13. https://doi.org/10.1038/s41699-023-0409-w
Salmi MS, Ahmed U, Aslfattahi N, Rahman S, Hardy JG, Anwar A (2022) Potent antibacterial activity of MXene-functionalized graphene nanocomposites. RSC Adv 12(51):33142–33155. https://doi.org/10.1039/D2RA04944A
Santos X et al (2022) Antibacterial capability of MXene (Ti3C2Tx) to produce PLA active contact surfaces for food packaging applications. Membranes Basel 12(11):1146. https://doi.org/10.3390/MEMBRANES12111146
Rasool K, Helal M, Ali A, Ren CE, Gogotsi Y, Mahmoud KA (2016) Antibacterial activity of Ti3C2Tx MXene. ACS Nano 10(3):3674–3684. https://doi.org/10.1021/ACSNANO.6B00181
Shan G, Ding Z, Gogotsi Y (2023) Two-dimensional MXenes and their applications. Front Phys. https://doi.org/10.1007/s11467-022-1254-2
Acknowledgements
This research was supported by Horizon Europe MSCA-2021-SE-01 project MX-MAP (#101086184) and H2020-MSCA-RISE-2019 SALSETH (#872370) and received support from the Ministry of Education and Science of Ukraine (0122U000784).
Author information
Authors and Affiliations
Contributions
K.D. - Conceptualisation, membrane preparation, cell culture, wrote the manuscript; Y. H. - contact angle, 3D visualization; W. S. - Raman, 3D visualization; V. K. - bacteriology experiment; B. Petrovic - conductivity measurements, SEM; A. R. - SEM of bacteria, 3D visualization; A. Stolarczyk - FTIR; N.Waloszczyk - Raman; I. Yanko - SEM of bacteria, figure preparation; K. Jekabsons - cell fluorescent microscopy; M. Čaplovičová - HRTEM; A.D.P. - HRTEM and manuscript writing; V. Z. - MXene preparation; O. Gogotsi -MAX-phase synthesis; I.Roslyk - MXene synthesis and delamination; I. Baginskiy - membrane impregnation of MXene; M. Radovic - electroconductivity measurement; S. Kojic - electroconductivity description; U. R. -conceptualization of biological experiment, manuscript writing; M. P. - supervision. manuscript writing and correction. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicting interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diedkova, K., Husak, Y., Simka, W. et al. Novel electrically conductive electrospun PCL-MXene scaffolds for cardiac tissue regeneration. Graphene and 2D mater 9, 59–76 (2024). https://doi.org/10.1007/s41127-023-00071-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41127-023-00071-5