Abstract
Purpose
This study aims at investigating the impact of imaging modality, deformable image registration (DIR) algorithm, and dose accumulation interval in the estimation of the cumulative delivered dose distribution.
Methods
This analysis involves 453 fractions of 20 prostate cancer patients treated with IMRT using CT-on-rails (CTOR) or VMAT using cone beam CT (CBCT). Dosimetric comparisons between intensity-based and contour-based DIR were based on mean dose, D1cc and V50.
Results
In the case of CTOR, the deviation of the cumulative mean dose against the planned dose was 1.4 ± 2.1 Gy for bladder and − 3.2 ± 5.3 Gy for rectum for the intensity-based DIR and 1.5 ± 2.0 Gy and − 3.9 ± 2.7 Gy for the contour-based DIR. The respective values for V50 were 3.0 ± 3.2% for bladder and − 6.1 ± 18.3% for rectum for the intensity-based DIR and 3.2 ± 3.8% and − 8.9 ± 8.3% for the contour-based DIR. In the case of CBCT, the deviation of the cumulative mean dose against the planned dose was 14.5 ± 8.6 Gy for bladder and − 1.7 ± 10.6 Gy for rectum for the intensity-based DIR and − 15.2 ± 46.0 Gy and − 31.2 ± 52.4 Gy for the contour-based DIR. The respective values for V50 were 14.8 ± 8.4% for bladder and 56.0 ± 101.8% for rectum for the intensity-based DIR and − 25.7 ± 50.1% and − 39.0 ± 55.4% for the contour-based DIR.
Conclusion
The analysis showed that the contour-based DIR algorithm performed much better than the intensity-based DIR and CTOR image guidance was more consistent than CBCT. Those findings indicate that the estimations of the cumulative delivered dose distribution based on auto-segmentation algorithms are in general less consistent compared to manual contouring (especially when CBCT is used for image guidance).










Similar content being viewed by others
References
Mavroidis, P., Ferreira, B. C., Papanikolaou, N., Svensson, R., Kappas, C., Lind, B. K., & Brahme, A. (2006). Assessing the difference between planned and delivered intensity-modulated radiotherapy dose distributions based on radiobiological measures. Clinical Oncologia, 18, 529–538.
Veiga, C., McClelland, J., Mouinuddin, S., Lourenço, A., Ricketts, K., Annkah, J., Modat, M., Ourselin, S., D’Souza, D., & Royle, G. (2014). Toward adaptive radiotherapy for head and neck patients: Feasibility study on using CT-to-CBCT deformable registration for “dose of the day” calculations. Medical Physics, 41, 031703.
Boda-Heggemann, J., Lohr, F., Wenz, F., Flentje, M., & Guckenberger, M. (2011). kV cone-beam CT-based IGRT: A clinical review. Strahlentherapie und Onkologie, 187, 284–291.
Hamilton, C. S., & Ebert, M. A. (2005). Volumetric uncertainty in radiotherapy. Clinical Oncology (Royal College of Radiologist), 17, 456–464.
Kapatoes, J. M., Olivera, G. H., Ruchala, K. J., & Mackie, T. R. (2001). On the verification of the incident energy fluence in tomotherapy IMRT. Physics in Medical and Biology, 46, 2953–2965.
Kapatoes, J. M., Olivera, G. H., Ruchala, K. J., Smilowitz, J. B., Reckwerdt, P. J., & Mackie, T. R. (2001). A feasible method for clinical delivery verification and dose reconstruction in tomotherapy. Medical Physics, 28, 528–542.
Low, D. A., Mutic, S., Dempsey, J. F., Gerber, R. L., Bosch, W. R., Perez, C. A., & Purdy, J. A. (1998). Quantitative dosimetric verification of an IMRT planning and delivery system. Radiotherapy and Oncology, 49, 305–316.
Lujan, A. E., Balter, J. M., & Ten Haken, R. K. (2003). A method for incorporating organ motion due to breathing into 3D dose calculations in the liver: Sensitivity to variations in motion. Medical Physics, 30, 2643–2649.
Papanikolaou, N., Yan, Y., Penagaricano, J., & Ratanatharathorn, V. (2002). The impact of daily patient setup error and tissue inhomogeneity on PTV coverage and OAR avoidance using IMRT. Medical Physics, 29(6), 1286.
Webb, S. (2000). Intensity-modulated radiation therapy. IOP Publishing.
Weiss, E., Vorwerk, H., Richter, S., & Hess, C. F. (2003). Interfractional and intrafractional accuracy during radiotherapy of gynecologic carcinomas: A comprehensive evaluation using the ExacTrac system. International Journal of Radiation Oncology, Biology, Physics, 56, 69–79.
Schwartz, D. L., & Dong, L. (2011). Adaptive radiation therapy for head and neck cancer-can an old goal evolve into a new standard? Journal of Oncology, 2011, 690595.
Veiga, C., Lourenco, A. M., Mouinuddin, S., van Herk, M., Modat, M., Ourselin, S., Royle, G., & McClelland, J. R. (2015). Toward adaptive radiotherapy for head and neck patients: Uncertainties in dose warping due to the choice of deformable registration algorithm. Medical Physics, 42, 760–769.
Garcia-Molla, R., Marco-Blancas, N., Bonaque, J., Vidueira, L., López-Tarjuelo, J., & Perez-Calatayud, J. (2015). Validation of a deformable image registration produced by a commercial treatment planning system in head and neck. Physica Medica, 31, 219–223.
Kadoya, N., Fujita, Y., Katsuta, Y., Dobashi, S., Takeda, K., Kishi, K., Kubozono, M., Umezawa, R., Sugawara, T., Matsushita, H., & Jingu, K. (2014). Evaluation of various deformable image registration algorithms for thoracic images. Journal of Radiation Research, 55, 175–182.
Kirby, N., Chuang, C., Ueda, U., & Pouliot, J. (2013). The need for application-based adaptation of deformable image registration. Medical Physics, 40, 011702.
Takayama, Y., Kadoya, N., Yamamoto, T., Ito, K., Chiba, M., Fujiwara, K., Miyasaka, Y., Dobashi, S., Sato, K., Takeda, K., & Jingu, K. (2017). Evaluation of the performance of deformable image registration between planning CT and CBCT images for the pelvic region: Comparison between hybrid and intensity-based DIR. Journal of Radiation Research, 58, 567–571.
Ahunbay, E. E., Peng, C., Guang-Pei, C., Narayanan, S., Yu, C., Lawton, C., & Li, X. A. (2008). An on-line re-planning scheme for inter-fractional variations. Medical Physics, 35, 3607–3615.
Ahunbay, E. E., Peng, C., Holmes, S., Godley, A., Lawton, C., & Li, X. A. (2010). An online adaptive replanning method for prostate radiotherapy. International Journal of Radiation Oncology Biology Physics, 77, 1561–1572.
Liu, F., Ahunbay, E., Lawton, C., & Li, X. A. (2014). Assessment and management of interfractional variations in daily diagnostic-quality-CT guided prostate-bed irradiation after prostatectomy. Medical Physics, 41, 031710.
Thor, M., Petersen, J. B. B., Bentzen, L., Bentzen, L., Høyer, M., & Muren, L. P. (2011). Deformable image registration for contour propagation from CT to cone-beam CT scans in radiotherapy of prostate cancer. Acta Oncologia, 50, 918–925.
Yan, D., Lockman, D., Brabbins, D., Tyburski, L., & Martinez, A. (2000). An off-line strategy for constructing a patient-specific planning target volume in adaptive treatment process for prostate cancer. International Journal of Radiation Oncology, Biology, Physics, 48, 289–302.
Zambrano, V., Furtado, H., Fabri, D., Lütgendorf-Caucig, C., Góra, J., Stock, M., Mayer, R., Birkfellner, W., & Georg, D. (2013). Performance validation of deformable image registration in the pelvic region. Journal of Radiation Research, 54, i120–i128.
Stroom, J. C., & Heijmen, B. J. (2002). Geometrical uncertainties, radiotherapy planning margins, and the ICRU-62 report. Radiotherapy and Oncologia, 64, 75–83.
Hammers, J., Pirozzi, S., Lindsay, D., Kaidar-Person, O., Tan, X., Chen, R. C., Das, S. K., & Mavroidis, P. (2020). Evaluation of a commercial DIR platform for contour propagation in prostate cancer patients treated with IMRT/VMAT. Journal of Applied Clinical Medical Physics, 21, 14–25.
Chetty, I. J. & Rosu-Bubulac, M. (2019). Deformable registration for dose accumulation. In Seminars in radiation oncology (Vol. 29, No. 3, pp. 198–208). WB Saunders.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical Approval
This article contains retrospective analysis of anonymized patient data.
Appendix
Appendix
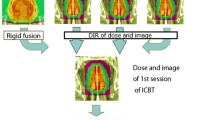
In the results presented in this work, the estimated delivered dose distributions are subject to the uncertainties of (1) calculating the fractional dose distribution to the IGRT scan vs. using the scaled planned dose distribution, which is rigidly translated to the IGRT scan; and (2) the DIR of the examined algorithms. The first source of uncertainty is discussed in point is discussed below. Regarding the second source of uncertainty, its magnitude was estimated through the analysis shown in Figs. 11 and 12. The variations in the fractional dose delivered to bladder and rectum were substantial in many patients due to their deformations (different bladder and rectal fillings) compared to the planned contours (see Fig. 11). Those DVHs are not subject to any of the uncertainties mentioned above. However, when the cumulative dose distributions get calculated from all the fractions the accuracy of the DIR comes into play. One way to estimate this accuracy is by comparing the deformed contours of bladder and rectum with the original planning contours. If the DIR accuracy is low, it will lead to deformed contours that are considerable different than the planning contours and this discrepancy will be reflected on the DVHs. In Fig. 12, it is shown that for the impact of this uncertainty for the contour-based (CBNIB) DIR on the DVHs is very small. As it was shown in another work by this group [25], the accuracy of the intensity-based DIR is lower (especially for the CBCT imaging modality) and affects more the estimated cumulative DVHs. For this reason, the estimated delivered DVHs that were calculated using the CBNIB DIR on CTOR images are considered as our reference in those comparisons.
Illustration of dose volume histograms of bladder (left) and rectum (right), which were derived by the planned dose distribution on the fractional IGRT contours, which were deformed to the planning CT using the contour-based DIR (solid lines) and the original planning contours (dashed lines). In this example, the DVHs from 36 different fractions throughout the course of the treatment are compared. For both bladder and rectum, the DVH differences are very small
In this work, the reason of using rigid registration to translate the planned dose distribution to each IGRT scan instead of calculating the dose distribution was mainly practical. The authors aimed to establish this workflow clinically and automation is a key point in this process. To calculate the dose distribution to each IGRT scan required much most manual work and time. In the case of CBCT, the poorer image quality would also have some impact in the accuracy of dose calculation. However, in order to examine the impact of any deformations of the external contour of the patient during the course of the treatment we performed a comparison between two workflows for different fractions of a given patients and the results are shown in Fig. 13. As it can be seen, the differences are minimal and much smaller in order of magnitude than the differences we observe due to the inter-fractional bladder and rectum deformations.
Illustration of dose volume histograms (DVHs) of bladder (left) and rectum (right), which were derived by the dose distributions that were calculated on the IGRT scans (solid lines) or were rigidly translated to the IGRT scan from the planning CT (dashed lines). In this example, the DVHs from seven different fractions throughout the course of the treatment are compared. For both bladder and rectum, the DVH differences are small
Rights and permissions
About this article
Cite this article
Hammers, J., Pirozzi, S., Narayanasamy, G. et al. Evaluation of the Dose Delivery Consistency and Its Dependence on Imaging Modality and Deformable Image Registration Algorithm in Prostate Cancer Patients. J. Med. Biol. Eng. 42, 74–86 (2022). https://doi.org/10.1007/s40846-021-00673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-021-00673-5