Abstract
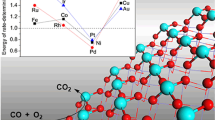
MXene is a variety of new two-dimensional (2D) materials with early transition metal carbides, nitrides, and carbonitrides. Quantum chemical studies have been carried out on the geometries, electronic structures, stability and catalytic properties of a non-noble metal single-atom catalyst (SAC) with single Co atom anchored on MXene materials of Mo2CS2. The Co adatom anchored on top of the Mo atom of this MXene is found to be rather stable, and this SAC is appropriate for CO oxidation. The charge transfers from the surface to the adsorbed CO and O2 play a significant role in the activation of these molecules on Co1/Mo2CS2. With this catalyst, the Eley-Rideal (ER), Langmuir-Hinshelwood (LH), and Termolecular Eley-Rideal (TER) mechanisms are explored for CO oxidation. We find that, while all the three mechanisms are feasible at low temperature, Co1/Mo2CS2 possesses higher catalytic activity for CO oxidation through the TER mechanism that features an intriguing OC(OO)CO intermediate (IM) adsorbed on Co single atom. The calculated activation energy barriers of the rate-limiting step are 0.67 eV (TER), 0.78 eV (LH) and 0.88 eV (ER), respectively. The present study illustrates that it is promising to develop and design low-cost, non-noble metal SACs using MXene types of 2D materials.

摘要
MXene是一类由前过渡态金属碳化物、氮化物或碳氮化物 构成的新型二维材料. 我们利用量子化学方法研究了单原子Co在 新型二维MXene材料Mo2CS2上的吸附构型、稳定性和催化性质. 研究发现Co原子可以稳定锚定在MXene材料的表面, 形成的单原 子催化剂适合于催化低温CO氧化. 计算表明, 吸附的CO和O2分子 与Co1/Mo2CS2催化剂表面之间的电荷转移在活化这些小分子时起 着重要作用. 我们研究了Co1/Mo2CS2催化氧化CO的三种机理: Eley-Rideal (ER), Langmuir-Hinshelwood (LH)和Termolecular Eley-Rideal (TER), 发现在低温下这三种反应机理都是可行的. 其 中在Co1/Mo2CS2催化剂上TER机理具有最高的催化活性, 计算的决 速步能垒分别为0.67 (TER), 0.78 (LH)和0.88 eV (ER). 我们的研究 结果表明, 利用二维材料MXene发展和设计经济的、非贵金属单 原子催化剂具有重要应用前景.
Similar content being viewed by others
References
Bunluesin T, Cordatos H, Gorte RJ. Study of CO oxidation kinetics on Rh/ceria. J Catal, 1995, 157: 222–226
Gong XQ, Liu ZP, Raval R, et al. A systematic study of CO oxidation on metals and metal oxides: Density functional theory calculations. J Am Chem Soc, 2004, 126: 8–9
Falsig H, Hvolbæk B, Kristensen I, et al. Trends in the catalytic CO oxidation activity of nanoparticles. Angew Chem, 2008, 120: 4913–4917
Lim FCH, Zhang J, Jin H, et al. A density functional theory study of CO oxidation on Pd-Ni alloy with sandwich structure. Appl Catal A-Ceneral, 2013, 451: 79–85
Esrafili MD, Saeidi N. Sn-embedded graphene: An active catalyst for CO oxidation to CO2? Phys E-Low-dimensional Syst NanoStruct, 2015, 74: 382–387
Alavi A, Hu P, Deutsch T, et al. CO oxidation on Pt(111): An ab initio density functional theory study. Phys Rev Lett, 1998, 80: 3650–3653
Chen MS, Cai Y, Yan Z, et al. Highly active surfaces for CO oxidation on Rh, Pd, and Pt. Surf Sci, 2007, 601: 5326–5331
Liu L, Zhou F, Wang L, et al. Low-temperature CO oxidation over supported Pt, Pd catalysts: Particular role of FeOx support for oxygen supply during reactions. J Catal, 2010, 274: 1–10
Stuve EM, Madix RJ, Brundle CR. CO oxidation on Pd(100): A study of the coadsorption of oxygen and carbon monoxide. Surf Sci, 1984, 146: 155–178
Zhang CJ, Hu P. CO oxidation on Pd(100) and Pd(111): A comparative study of reaction pathways and reactivity at low and medium coverages. J Am Chem Soc, 2001, 123: 1166–1172
Molina LM, Hammer B. Active role of oxide support during CO oxidation at Au/MgO. Phys Rev Lett, 2003, 90: 206102
Su HY, Yang MM, Bao XH, et al. The effect of water on the CO oxidation on Ag(111) and Au(111) surfaces: A first-principle study. J Phys Chem C, 2008, 112: 17303–17310
Ghosh TK, Nair NN. Rh1/γ-Al2O3 single-atom catalysis of O2 activation and CO oxidation: Mechanism, effects of hydration, oxidation state, and cluster size. ChemCatChem, 2013, 5: 1811–1821
Zhang LL, Sun MJ, Liu CG. CO oxidation on the phosphotungstic acid supported Rh single-atom catalysts via Rh-assisted Mars-van Krevelen mechanism. Mol Catal, 2019, 462: 37–45
Gao F, Goodman DW. Model catalysts: Simulating the complexities of heterogeneous catalysts. Annu Rev Phys Chem, 2012, 63: 265–286
Liu JC, Wang YG, Li J. Toward rational design of oxide-supported single-atom catalysts: Atomic dispersion of gold on ceria. J Am Chem Soc, 2017, 139: 6190–6199
Li J, Li Y, Zhang T. Recent progresses in the research of singleatom catalysts. Sci China Mater, 2020, 63: 889–891
Li J, Liu J, Zhang T. Preface to the special issue of the international symposium on single-atom catalysis (ISSAC-2016). Chin J Catal, 2017, 38: 1431
Zhang X, Lei J, Wu D, et al. A Ti-anchored Ti2CO2 monolayer (MXene) as a single-atom catalyst for CO oxidation. J Mater Chem A, 2016, 4: 4871–4876
Heiz U, Sanchez A, Abbet S, et al. Catalytic oxidation of carbon monoxide on monodispersed platinum clusters: Each atom counts. J Am Chem Soc, 1999, 121: 3214–3217
Lin J, Qiao B, Liu J, et al. Design of a highly active Ir/Fe(OH)x catalyst: Versatile application of Pt-group metals for the preferential oxidation of carbon monoxide. Angew Chem, 2012, 124: 2974–2978
Lei Y, Mehmood F, Lee S, et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science, 2010, 328: 224–228
Qiao B, Wang A, Yang X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem, 2011, 3: 634–641
Yang XF, Wang A, Qiao B, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc Chem Res, 2013, 46: 1740–1748
Wang A, Li J, Zhang T. Heterogeneous single-atom catalysis. Nat Rev Chem, 2018, 2: 65–81
Liu JC, Tang Y, Wang YG, et al. Theoretical understanding of the stability of single-atom catalysts. Natl Sci Rev, 2018, 5: 638–641
Li Z, Chen Y, Ji S, et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host-guest strategy. Nat Chem, 2020, 12: 764–772
Zhao S, Chen F, Duan S, et al. Remarkable active-site dependent H2O promoting effect in CO oxidation. Nat Commun, 2019, 10: 3824
He Y, Liu JC, Luo L, et al. Size-dependent dynamic structures of supported gold nanoparticles in CO oxidation reaction condition. Proc Natl Acad Sci USA, 2018, 115: 7700–7705
Liang J, Lin J, Liu J, et al. Dual metal active sites in an Ir1/FeOx single-atom catalyst: A redox mechanism for the water-gas shift reaction. Angew Chem Int Ed, 2020, 59: 12868–12875
Wang L, Huang L, Liang F, et al. Preparation, characterization and catalytic performance of single-atom catalysts. Chin J Catal, 2017, 38: 1528–1539
Chen Y, Ji S, Chen C, et al. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule, 2018, 2: 1242–1264
Sun S, Zhang G, Gauquelin N, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci Rep, 2013, 3: 1775
Yan H, Cheng H, Yi H, et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene. J Am Chem Soc, 2015, 137: 10484–10487
Thang HV, Pacchioni G, DeRita L, et al. Nature of stable single atom Pt catalysts dispersed on anatase TiO2. J Catal, 2018, 367: 104–114
Li X, Bi W, Zhang L, et al. Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution. Adv Mater, 2016, 28: 2427–2431
Nie L, Mei D, Xiong H, et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science, 2017, 358: 1419–1423
Deng D, Novoselov KS, Fu Q, et al. Catalysis with two-dimensional materials and their heterostructures. Nat Nanotech, 2016, 11: 218–230
Chia X, Pumera M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat Catal, 2018, 1: 909–921
Anasori B, Lukatskaya MR, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater, 2017, 2: 16098
Li WL, Chen X, Jian T, et al. From planar boron clusters to borophenes and metalloborophenes. Nat Rev Chem, 2017, 1: 71
Zhang XL, Yamada H, Saito T, et al. Development of hydrogenselective triphenylmethoxysilane-derived silica membranes with tailored pore size by chemical vapor deposition. J Membrane Sci, 2016, 499: 28–35
Wang M, Wang Z. Single Ni atom incorporated with pyridinic nitrogen graphene as an efficient catalyst for CO oxidation: First-principles investigation. RSC Adv, 2017, 7: 48819–48824
Wu P, Du P, Zhang H, et al. Graphyne-supported single Fe atom catalysts for CO oxidation. Phys Chem Chem Phys, 2015, 17: 1441–1449
Lin ZZ. Graphdiyne-supported single-atom Sc and Ti catalysts for high-efficient CO oxidation. Carbon, 2016, 108: 343–350
Yin CG, Ma Y, Liu ZJ, et al. Multifunctional boron nitride nanosheet/polymer composite nanofiber membranes. Polymer, 2019, 162: 100–107
Zeng H, Zhi C, Zhang Z, et al. “White graphenes”: Boron nitride nanoribbons via boron nitride nanotube unwrapping. Nano Lett, 2010, 10: 5049–5055
Li M, Li Y, Zhou Z, et al. Metal-decorated defective BN nanosheets as hydrogen storage materials. Front Phys, 2011, 6: 224–230
Liu G, Lei XL, Wu MS, et al. Comparison of the stability of freestanding silicene and hydrogenated silicene in oxygen: A first principles investigation. J Phys-Condens Matter, 2014, 26: 355007
Bianco E, Butler S, Jiang S, et al. Stability and exfoliation of germanane: A germanium graphane analogue. ACS Nano, 2013, 7: 4414–4421
Dávila ME, Xian L, Cahangirov S, et al. Germanene: A novel two-dimensional germanium allotrope akin to graphene and silicene. New J Phys, 2014, 16: 095002
Lin JH, Zhang H, Cheng XL. First-principle study on the optical response of phosphorene. Front Phys, 2015, 10: 1–9
Zhu Z, Chen C, Liu J, et al. The electronic and optical properties of Au doped single-layer phosphorene. Russ J Phys Chem, 2018, 92: 132–139
Mao J, Wang Y, Zheng Z, et al. The rise of two-dimensional MoS2 for catalysis. Front Phys, 2018, 13: 138118
Lukowski MA, Daniel AS, Meng F, et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J Am Chem Soc, 2013, 135: 10274–10277
Wang T, Andrews K, Bowman A, et al. High-performance WSe2 phototransistors with 2D/2D Ohmic contacts. Nano Lett, 2018, 18: 2766–2771
Allain A, Kis A. Electron and hole mobilities in single-layer WSe2. ACS Nano, 2014, 8: 7180–7185
Huang X, Zhang H. Molecular crystals on two-dimensional van der Waals substrates. Sci China Mater, 2015, 58: 5–8
Ahmed S, Yi J. Two-dimensional transition metal dichalcogenides and their charge carrier mobilities in field-effect transistors. Nano-Micro Lett, 2017, 9: 50
Naguib M, Come J, Dyatkin B, et al. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem Commun, 2012, 16: 61–64
Naguib M, Kurtoglu M, Presser V, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater, 2011, 23: 4248–4253
Naguib M, Mochalin VN, Barsoum MW, et al. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv Mater, 2014, 26: 992–1005
Lin Z, Barbara D, Taberna PL, et al. Capacitance of Ti3C2Tx MXene in ionic liquid electrolyte. J Power Sources, 2016, 326: 575–579
Wang L, Zhang H, Wang B, et al. Synthesis and electrochemical performance of Ti3C2Tx with hydrothermal process. Electron Mater Lett, 2016, 12: 702–710
Lei JC, Zhang X, Zhou Z. Recent advances in MXene: Preparation, properties, and applications. Front Phys, 2015, 10: 276–286
Li K, Jiao T, Xing R, et al. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci China Mater, 2018, 61: 728–736
Lin H, Chen L, Lu X, et al. Two-dimensional titanium carbide MXenes as efficient non-noble metal electrocatalysts for oxygen reduction reaction. Sci China Mater, 2019, 62: 662–670
Guo Z, Zhou J, Zhu L, et al. MXene: A promising photocatalyst for water splitting. J Mater Chem A, 2016, 4: 11446–11452
Fan Z, Wang Y, Xie Z, et al. Modified MXene/holey graphene films for advanced supercapacitor electrodes with superior energy storage. Adv Sci, 2018, 5: 1800750
Zhang Y, Zhan R, Xu Q, et al. Circuit board-like CoS/MXene composite with superior performance for sodium storage. Chem Eng J, 2019, 357: 220–225
Cheng C, Zhang X, Wang M, et al. Single Pd atomic catalyst on Mo2CO2 monolayer (MXene): Unusual activity for CO oxidation by trimolecular Eley-Rideal mechanism. Phys Chem Chem Phys, 2018, 20: 3504–3513
Zhang J, Zhao Y, Guo X, et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat Catal, 2018, 1: 985–992
Zhao D, Chen Z, Yang W, et al. MXene (Ti3C2) vacancy-confined single-atom catalyst for efficient functionalization of CO2. J Am Chem Soc, 2019, 141: 4086–4093
Chen Z, Huang S, Huang B, et al. Transition metal atoms implanted into MXenes (M2CO2) for enhanced electrocatalytic hydrogen evolution reaction. Appl Surf Sci, 2020, 509: 145319
Zhu J, Ha E, Zhao C, et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord Chem Rev, 2017, 352: 306–327
Shen Z, Fan X, Ma S, et al. 3d transitional-metal single atom catalysis toward hydrogen evolution reaction on MXenes supports. Int J Hydrogen Energy, 2020, 45: 14396–14406
Zhang X, Zhang Z, Zhou Z. MXene-based materials for electrochemical energy storage. J Energy Chem, 2018, 27: 73–85
Kresse C, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci, 1996, 6: 15–50
Maniopoulou A, Davidson ERM, Grau-Crespo R, et al. Introducing k-point parallelism into VASP. Comput Phys Commun, 2012, 183: 1696–1701
Torrent M, Holzwarth NAW, Jollet F, et al. Electronic structure packages: Two implementations of the projector augmented wave (PAW) formalism. Comput Phys Commun, 2010, 181: 1862–1867
Torrent M, Jollet F, Bottin F, et al. Implementation of the projector augmented-wave method in the ABINIT code: Application to the study of iron under pressure. Comput Mater Sci, 2008, 42: 337–351
Jollet F, Torrent M, Holzwarth N. Generation of projector augmented-wave atomic data: A 71 element validated table in the XML format. Comput Phys Commun, 2014, 185: 1246–1254
Zhang Y, Yang W. Comment on “generalized gradient approximation made simple”. Phys Rev Lett, 1998, 80: 890
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 77: 3865–3868
Yu M, Trinkle DR. Accurate and efficient algorithm for Bader charge integration. J Chem Phys, 2011, 134: 064111
Tang W, Sanville E, Henkelman G. A grid-based Bader analysis algorithm without lattice bias. J Phys-Condens Matter, 2009, 21: 084204
Zarkevich NA, Johnson DD. Nudged-elastic band method with two climbing images: Finding transition states in complex energy landscapes. J Chem Phys, 2015, 142: 024106
Henkelman C, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys, 2000, 113: 9901–9904
Kästner J, Sherwood P. Superlinearly converging dimer method for transition state search. J Chem Phys, 2008, 128: 014106
Henkelman C, Jónsson H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J Chem Phys, 1999, 111: 7010–7022
Talib SH, Yu X, Yu Q, et al. Non-noble metal single-atom catalysts with phosphotungstic acid (PTA) support: A theoretical study of ethylene epoxidation. Sci China Mater, 2020, 63: 1003–1014
Qiao B, Liang JX, Wang A, et al. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res, 2015, 8: 2913–2924
Wang YG, Yoon Y, Glezakou VA, et al. The role of reducible oxide-metal cluster charge transfer in catalytic processes: New insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J Am Chem Soc, 2013, 135: 10673–10683
Wang YG, Mei D, Glezakou VA, et al. Dynamic formation of single-atom catalytic active sites on ceria-supported gold nanoparticles. Nat Commun, 2015, 6: 6511
Tang Y, Asokan C, Xu M, et al. Rh single atoms on TiO2 dynamically respond to reaction conditions by adapting their site. Nat Commun, 2019, 10: 4488
Chatt J, Duncanson LA. 586. Olefin co-ordination compounds. Part III. Infra-red spectra and structure: Attempted preparation of acetylene complexes. J Chem Soc, 1953: 2939–2947
Chatt J, Duncanson LA, Venanzi LM. Directing effects in inorganic substitution reactions. Part I. A hypothesis to explain the trans-effect. J Chem Soc, 1955: 4456–4460
Mao K, Li L, Zhang W, et al. A theoretical study of single-atom catalysis of CO oxidation using Au embedded 2D h-BN monolayer: A CO-promoted O2 activation. Sci Rep, 2014, 4: 5441
Lu Z, Lv P, Xue J, et al. Pd1/BN as a promising single atom catalyst of CO oxidation: A dispersion-corrected density functional theory study. RSC Adv, 2015, 5: 84381–84388
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21590792, 91426302, and 21433005), and Guangdong Provincial Key Laboratory of Catalysis (2020B121201002). Yu X thanks the National Science Basic Research Program of Shaanxi Province (2019JM-226). Muhammad S is grateful to the financial and technical support from the Research Center for Advanced Materials Science (RCAMS) at King Khalid University through the Grant (RCAMS/KKU/014-20). The calculations were performed using supercomputers at Tsinghua National Laboratory for Information Science and Technology.
Author information
Authors and Affiliations
Contributions
Li J designed the project. Talib SH and Yu X performed the calculations. All authors discussed and interpreted the data. Talib SH, Yu X and Li J co-wrote and revised the manuscript.
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Computational details and supporting data are available in the online version of the paper.
Shamraiz Hussain Talib received a PhD degree at the Department of Chemistry and Key Laboratory of Organic Optoelectronics & Molecular Engineering, Tsinghua University, under the supervision of Prof. Jun Li. He received his M. Phil degree (majored in chemistry) from the Department of Chemistry, Mohi-Ud-Din Islamic University, AJ&K, Pakistan in 2016. His PhD research focuses on the theoretical investigations on heterogeneous single-atom catalysts.
Xiaohu Yu received his PhD degree from the Institute of Coal Chemistry, Chinese Academy of Sciences in 2013. He did postdoctoral research at Moscow Institute of Physics and Technology from 2013 to 2015. He worked as visiting scholar in Prof. Jun Li’s group at Tsinghua University from 2019 to 2020. He is now an associate professor at Shaanxi University of Technology. His research interests focus on theoretical inorganic chemistry and computational catalysis science.
Jun Li received his PhD degree from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences in 1992. He did postdoctoral research at the University of Siegen and The Ohio State University from 1994 to 1997. He worked as a research scientist at The Ohio State University and a senior research scientist at the Pacific Northwest National Laboratory from 1997 to 2009. He is now a full professor at Tsinghua University. His research involves theoretical chemistry, heavy-element chemistry, and computational catalysis science.
Supplementary information
Rights and permissions
About this article
Cite this article
Talib, S.H., Baskaran, S., Yu, X. et al. Non-noble metal single-atom catalyst of Co1/MXene (Mo2CS2) for CO oxidation. Sci. China Mater. 64, 651–663 (2021). https://doi.org/10.1007/s40843-020-1458-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1458-5