Abstract
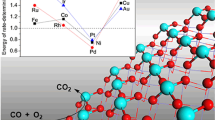
We report herein a new sulfur-functionalized MXene Ti2C (Ti2CS2)-supported osmium-metal single-atom catalyst (SAC) Os1/Ti2CS2 with high low-temperature catalytic activity for CO oxidation. Using periodic density functional theory calculations, the most stable SAC, Os1/Ti2CS2, has been screened from a series of group 8–11 transition metal SACs M1/Ti2CS2 (M = Fe, Co, Ni, Cu; Ru, Rh, Pd, Ag; Os, Ir, Pt, Au). The calculations show that it is favorable for O2 and CO to be coadsorbed on the Os1 single atom (SA) of Os1/Ti2CS2 and the adsorption energy of the first O2 molecule is slightly higher than that of CO. Moreover, the termolecular co-adsorption of O2 + 2CO on Os1 SA is also possible, which is favorable for CO oxidation on Os1 SA through a novel three-molecule reaction mechanism. Accordingly, four different catalytic mechanisms, the Langmuir-Hinshelwood (L-H), Eley-Rideal (E-R), termolecular Langmuir-Hinshelwood-A (TLH-A) and termolecular Langmuir-Hinshelwood-B (TLH-B), are systematically studied for CO oxidation by O2 on Os1/Ti2CS2. The theoretical studies indicate that the TLH-B mechanism is the most feasible for CO oxidation with the reaction barrier energy of only 0.74 eV, which is far lower than for L-H, E-R and TLH-A with barrier energies of 1.06, 1.09 and 1.47 eV, respectively. The results provide fundamental understanding to the surface chemistry of MXene and designing new sulfur-functionalized two-dimensional MXene catalytic nanomaterials.

摘要
本文报道了一种对CO氧化反应具有低温催化活性的新型硫功能 化MXene-Ti2C (Ti2CS2)负载的锇金属单原子催化剂Os1/Ti2CS2. 通过密 度泛函理论计算, 从一系列过渡金属(M = Fe, Co, Ni, Cu; Ru, Rh, Pd, Ag; Os, Ir, Pt, Au)中筛选出最稳定的锇金属单原子催化剂. 计算结果表 明, Os1/Ti2CS2有利于O2和CO的共吸附, 且O2分子的吸附能略高于CO 分子. 此外, 由于O2+2CO能稳定地共吸附在Os1单原子上, CO氧化反应 可能通过三分子反应机理进行. 因此, 我们研究了CO在Os1/Ti2CS2单原 子催化剂上发生氧化反应的四种不同的催化机理: Langmuir-Hinshelwood (L–H)、Eley Rideal (E–R)、termolecular Langmuir-Hinshelwood-A (TLH-A)和termolecular Langmuir-Hinshelwood-B (TLH-B)机 理. 结果表明TLH-B机理最可能发生, 其反应势垒仅为0.74 eV, 远低于 L–H、E–R和TLH-A的反应势垒(分别为1.06, 1.09和1.47 eV). 上述研究 结果有助于理解MXene的表面化学并设计稳定的新型二维硫端基 MXene催化材料.
Similar content being viewed by others
References
Qiao B, Wang A, Yang X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem, 2011, 3: 634–641
Chen YJ, Zhuo HY, Pan Y, et al. Triazine COF-supported single-atom catalyst (Pd1/trzn-COF) for CO oxidation. Sci China Mater, 2021, 64: 1939–1951
Zhu C, Liang JX, Meng Y, et al. Mn-corrolazine-based 2D-nanocatalytic material with single Mn atoms for catalytic oxidation of alkane to alcohol. Chin J Catal, 2021, 42: 1030–1039
Nie G, Li P, Liang JX, et al. Theoretical investigation on the photocatalytic activity of the Au/g-C3N4 monolayer. J Theor Comput Chem, 2017, 16: 1750013
Liu Y, Liu JC, Li TH, et al. Unravelling the enigma of nonoxidative conversion of methane on iron single-atom catalysts. Angew Chem Int Ed, 2020, 59: 18586–18590
Tang Y, Wang YG, Li J. Theoretical investigations of Pt1@CeO2 singleatom catalyst for CO oxidation. J Phys Chem C, 2017, 121: 11281–11289
Zhang M, Wang YG, Chen W, et al. Metal (hydr)oxides@polymer core-shell strategy to metal single-atom materials. J Am Chem Soc, 2017, 139: 10976–10979
Zhang N, Zhang X, Tao L, et al. Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew Chem Int Ed, 2021, 60: 6170–6176
Zhu M, Zhao C, Liu X, et al. Single atomic cerium sites with a high coordination number for efficient oxygen reduction in proton-exchange membrane fuel cells. ACS Catal, 2021, 11: 3923–3929
Huang CX, Li G, Yang LM, et al. Ammonia synthesis using single-atom catalysts based on two-dimensional organometallic metal phthalocyanine monolayers under ambient conditions. ACS Appl Mater Interfaces, 2021, 13: 608–621
Yang XF, Wang A, Qiao B, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc Chem Res, 2013, 46: 1740–1748
Wang A, Li J, Zhang T. Heterogeneous single-atom catalysis. Nat Rev Chem, 2018, 2: 65–81
Zhuo HY, Zhang X, Liang JX, et al. Theoretical understandings of graphene-based metal single-atom catalysts: Stability and catalytic performance. Chem Rev, 2020, 120: 12315–12341
Liu JC, Tang Y, Wang YG, et al. Theoretical understanding of the stability of single-atom catalysts. Natl Sci Rev, 2018, 5: 638–641
Liu K, Tang Y, Yu Z, et al. High-loading and thermally stable Pt1/MgAl1.2Fe0.8O4 single-atom catalysts for high-temperature applications. Sci China Mater, 2020, 63: 949–958
Wei H, Liu X, Wang A, et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat Commun, 2014, 5: 5634
Liu S, Yang HB, Hung SF, et al. Elucidating the electrocatalytic CO2 reduction reaction over a model single-atom nickel catalyst. Angew Chem Int Ed, 2020, 59: 798–803
Liu JC, Wang YG, Li J Toward rational design of oxide-supported single-atom catalysts: Atomic dispersion of gold on ceria. J Am Chem Soc, 2017, 139: 6190–6199
Xing DH, Xu CQ, Wang YG, et al. Heterogeneous single-cluster catalysts for selective semihydrogenation of acetylene with graphdiynesupported triatomic clusters. J Phys Chem C, 2019, 123: 10494–10500
Liu W, Chen Y, Qi H, et al. A durable nickel single-atom catalyst for hydrogenation reactions and cellulose valorization under harsh conditions. Angew Chem Int Ed, 2018, 57: 7071–7075
Yang J, Wang X, Qu Y, et al. Bi-based metal-organic framework derived leafy bismuth nanosheets for carbon dioxide electroreduction. Adv Energy Mater, 2020, 10: 2001709
Yang J, Wang Z, Huang CX, et al. Compressive strain modulation of single iron sites on helical carbon support boosts electrocatalytic oxygen reduction. Angew Chem Int Ed, 2021, 60: 22722–22728
Wang X, Yang LM. Efficient modulation of the catalytic performance of electrocatalytic nitrogen reduction with transition metals anchored on N/O-codoped graphene by coordination engineering. J Mater Chem A, 2022, doi: https://doi.org/10.1039/D1TA08877G
Wang YG, Mei D, Glezakou VA, et al. Dynamic formation of singleatom catalytic active sites on ceria-supported gold nanoparticles. Nat Commun, 2015, 6: 6511
Ma XL, Liu JC, Xiao H, et al. Surface single-cluster catalyst for N2-to-NH3 thermal conversion. J Am Chem Soc, 2018, 140: 46–49
Liu JC, Ma XL, Li Y, et al. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat Commun, 2018, 9: 1610
Wang L, Huang L, Liang F, et al. Preparation, characterization and catalytic performance of single-atom catalysts. Chin J Catal, 2017, 38: 1528–1539
Lin J, Wang A, Qiao B, et al. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J Am Chem Soc, 2013, 135: 15314–15317
Qiao B, Liang JX, Wang A, et al. Single atom gold catalysts for low-temperature CO oxidation. Chin J Catal, 2016, 37: 1580–1586
Ren Y, Tang Y, Zhang L, et al. Unraveling the coordination structure-performance relationship in Pt1/Fe2O3 single-atom catalyst. Nat Commun, 2019, 10: 4500
Zhuo HY, Yu X, Yu Q, et al. Selective hydrogenation of acetylene on graphene-supported non-noble metal single-atom catalysts. Sci China Mater, 2020, 63: 1741–1749
Chen Y, Ji S, Sun W, et al. Engineering the atomic interface with single platinum atoms for enhanced photocatalytic hydrogen production. Angew Chem Int Ed, 2020, 59: 1295–1301
Yin P, Yao T, Wu Y, et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew Chem Int Ed, 2016, 55: 10800–10805
Li Z, Ji S, Liu Y, et al. Well-defined materials for heterogeneous catalysis: From nanoparticles to isolated single-atom sites. Chem Rev, 2020, 120: 623–682
Xiong Y, Dong J, Huang ZQ, et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat Nanotechnol, 2020, 15: 390–397
Wei S, Li A, Liu JC, et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat Nanotech, 2018, 13: 856–861
Li X, Liu L, Ren X, et al. Microenvironment modulation of single-atom catalysts and their roles in electrochemical energy conversion. Sci Adv, 2020, 6: eabb6833
Xu H, Xu CQ, Cheng D, et al. Identification of activity trends for CO oxidation on supported transition-metal single-atom catalysts. Catal Sci Technol, 2017, 7: 5860–5871
Lang R, Xi W, Liu JC, et al. Non defect-stabilized thermally stable single-atom catalyst. Nat Commun, 2019, 10: 234
Talib SH, Yu X, Yu Q, et al. Non-noble metal single-atom catalysts with phosphotungstic acid (PTA) support: A theoretical study of ethylene epoxidation. Sci China Mater, 2020, 63: 1003–1014
Zhang Y, Zhang C, Mo Y, et al. Planar tetracoordinate silicon in organic molecules as carbenoid-type amphoteric centers: A computational study. Chem Eur J, 2021, 27: 1402–1409
Fang L, Zhang C, Cao X, et al. Tackling the inertness of CO2: Facile activation and electroreduction on the metal-free SiN4C4 monolayer sheet. J Phys Chem C, 2020, 124: 18660–18669
Long B, Tang Y, Li J. New mechanistic pathways for CO oxidation catalyzed by single-atom catalysts: Supported and doped Au1/ThO2. Nano Res, 2016, 9: 3868–3880
Xu J, Li R, Xu CQ, et al. Underpotential-deposition synthesis and inline electrochemical analysis of single-atom copper electrocatalysts. Appl Catal B-Environ, 2021, 289: 120028
Liang JX, Lin J, Liu J, et al. Dual metal active sites in an Ir1/FeOx singleatom catalyst: A redox mechanism for the water-gas shift reaction. Angew Chem Int Ed, 2020, 59: 12868–12875
Liang JX, Yang XF, Wang A, et al. Theoretical investigations of nonnoble metal single-atom catalysis: Ni1/FeOx for CO oxidation. Catal Sci Technol, 2016, 6: 6886–6892
Gao D, Liu T, Wang G, et al. Structure sensitivity in single-atom catalysis toward CO2 electroreduction. ACS Energy Lett, 2021, 6: 713–727
Wang Q, Huang X, Zhao ZL, et al. Ultrahigh-loading of Ir single atoms on NiO matrix to dramatically enhance oxygen evolution reaction. J Am Chem Soc, 2020, 142: 7425–7433
Baskaran S, Xu CQ, Wang YG, et al. Catalytic mechanism and bonding analyses of Au-Pd single atom alloy (SAA): CO oxidation reaction. Sci China Mater, 2020, 63: 993–1002
Ji S, Qu Y, Wang T, et al. Rare-earth single erbium atoms for enhanced photocatalytic CO2 reduction. Angew Chem Int Ed, 2020, 59: 10651–10657
Lei Y, Wang Y, Liu Y, et al. Designing atomic active centers for hydrogen evolution electrocatalysts. Angew Chem Int Ed, 2020, 59: 20794–20812
Liang J, Yang X, Xu C, et al. Catalytic ativities of single-atom catalysts for CO oxidation: Pt1/FeOxvs. Fe1/FeOx. Chin J Catal, 2017, 38: 1566–1573
Guo X, Fang G, Li G, et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science, 2014, 344: 616–619
Wang X, Yang LM. Unveiling the underlying mechanism of nitrogen fixation by a new class of electrocatalysts two-dimensional TM@g-C4N3 monosheets. Appl Surf Sci, 2022, 576: 151839
Cao X, Ji Y, Luo Y. Dehydrogenation of propane to propylene by a Pd/Cu single-atom catalyst: Insight from first-principles calculations. J Phys Chem C, 2015, 119: 1016–1023
Tang S, Zhou X, Liu T, et al. Single nickel atom supported on hybridized graphene-boron nitride nanosheet as a highly active bi-functional electrocatalyst for hydrogen and oxygen evolution reactions. J Mater Chem A, 2019, 7: 26261–26265
Wei Y, Soomro RA, Xie X, et al. Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D mxenes. J Energy Chem, 2021, 55: 244–255
Zhu Q, Li J, Simon P, et al. Two-dimensional MXenes for electrochemical capacitor applications: Progress, challenges and perspectives. Energy Storage Mater, 2021, 35: 630–660
Wang Y, Mao J, Meng X, et al. Catalysis with two-dimensional materials confining single atoms: Concept, design, and applications. Chem Rev, 2019, 119: 1806–1854
Cui X, Li H, Wang Y, et al. Room-temperature methane conversion by graphene-confined single iron atoms. Chem, 2018, 4: 1902–1910
Zhang M, Lai C, Li B, et al. Mxenes as superexcellent support for confining single atom: Properties, synthesis, and electrocatalytic applications. Small, 2021, 17: 2007113
Oschinski H, Morales-García Á, Illas F. Interaction of first row transition metals with M2C (M = Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W) MXenes: A quest for single-atom catalysts. J Phys Chem C, 2021, 125: 2477–2484
Wang S, Li J, Li Q, et al. Metal single-atom coordinated graphitic carbon nitride as an efficient catalyst for CO oxidation. Nanoscale, 2020, 12: 364–371
Kan D, Lian R, Wang D, et al. Screening effective single-atom ORR and OER electrocatalysts from Pt decorated MXenes by first-principles calculations. J Mater Chem A, 2020, 8: 17065–17077
Shayesteh Zeraati A, Mirkhani SA, Sun P, et al. Improved synthesis of Ti3C2Tx MXenes resulting in exceptional electrical conductivity, high synthesis yield, and enhanced capacitance. Nanoscale, 2021, 13: 3572–3580
Li K, Zhang S, Li Y, et al. MXenes as noble-metal-alternative co-catalysts in photocatalysis. Chin J Catal, 2021, 42: 3–14
Zhao MR, Song B, Yang LM. Two-dimensional single-atom catalyst TM3(HAB)2 monolayers for electrocatalytic dinitrogen reduction using hierarchical high-throughput screening. ACS Appl Mater Interfaces, 2021, 13: 26109–26122
Naguib M, Kurtoglu M, Presser V, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater, 2011, 23: 4248–4253
Pang J, Mendes RG, Bachmatiuk A, et al. Applications of 2D MXenes in energy conversion and storage systems. Chem Soc Rev, 2019, 48: 72–133
Lin H, Chen L, Lu X, et al. Two-dimensional titanium carbide MXenes as efficient non-noble metal electrocatalysts for oxygen reduction reaction. Sci China Mater, 2019, 62: 662–670
Wang C, Xu J, Wang Y, et al. MXene (Ti2NTx): Synthesis, characteristics and application as a thermo-optical switcher for all-optical wavelength tuning laser. Sci China Mater, 2021, 64: 259–265
Ye Y, Yi W, Liu W, et al. Remarkable surface-enhanced Raman scattering of highly crystalline monolayer Ti3C2 nanosheets. Sci China Mater, 2020, 63: 794–805
Zhang J, Zhao Y, Guo X, et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat Catal, 2018, 1: 985–992
Talib SH, Baskaran S, Yu X, et al. Non-noble metal single-atom catalyst of Co1/MXene (Mo2CS2) for CO oxidation. Sci China Mater, 2021, 64: 651–663
Yin J, Zhan F, Jiao T, et al. Facile preparation of self-assembled MXene@Au@Cds nanocomposite with enhanced photocatalytic hydrogen production activity. Sci China Mater, 2020, 63: 2228–2238
Li J, Yang QQ, Hu YX, et al. Design of lamellar Mo2C nanosheets assembled by Mo2C nanoparticles as an anode material toward excellent sodium-ion capacitors. ACS Sustain Chem Eng, 2019, 7: 18375–18383
Zhang X, Lei J, Wu D, et al. A Ti-anchored Ti2CO2 monolayer (MXene) as a single-atom catalyst for CO oxidation. J Mater Chem A, 2016, 4: 4871–4876
Naguib M, Come J, Dyatkin B, et al. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem Commun, 2012, 16: 61–64
Shein IR, Ivanovskii AL. Graphene-like titanium carbides and nitrides Tin+1CN, Tin+1Nn (n=1, 2, and 3) from de-intercalated max phases: First-principles probing of their structural, electronic properties and relative stability. Comput Mater Sci, 2012, 65: 104–114
Deysher G, Shuck CE, Hantanasirisakul K, et al. Synthesis of Mo4VAlC4 MAX phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals. ACS Nano, 2020, 14: 204–217
Kamysbayev V, Filatov AS, Hu H, et al. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science, 2020, 369: 979–983
Meng Q, Ma J, Zhang Y, et al. The S-functionalized Ti3C2 Mxene as a high capacity electrode material for Na-ion batteries: a DFT study. Nanoscale, 2018, 10: 3385–3392
Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys Rev B, 1993, 47: 558–561
Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B, 1999, 59: 1758–1775
Zhang Y, Yang W. Comment on “Generalized Gradient Approximation Made Simple”. Phys Rev Lett, 1998, 80: 890
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 77: 3865–3868
Liu X, Shao X, Li F, et al. Anchoring effects of S-terminated Ti2C MXene for lithium-sulfur batteries: A first-principles study. Appl Surf Sci, 2018, 455: 522–526
Siriwardane EMD, Demiroglu I, Sevik C, et al. Assessment of sulfurfunctionalized MXenes for Li-ion battery applications. J Phys Chem C, 2020, 124: 21293–21304
Henkelman G, Jónsson H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J Chem Phys, 1999, 111: 7010–7022
Liang J, Yu Q, Yang X, et al. A systematic theoretical study on FeOx-supported single-atom catalysts: M1/FeOx for CO oxidation. Nano Res, 2018, 11: 1599–1611
Pyykkö P, Atsumi M. Molecular single-bond covalent radii for elements 1-118. Chem Eur J, 2009, 15: 186–197
Vaska L. Dioxygen-metal complexes: toward a unified view. Acc Chem Res, 1976, 9: 175–183
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21963005, 21763006, 22033005 and 22038002), the Natural Science Foundation of Guizhou University ([2021]40 and [2020] 32) and Guangdong Provincial Key Laboratory of Catalysis (2020B121201002). The calculations were performed by using supercomputers at SUSTech and Shanghai Supercomputing Center. The authors are grateful to Dr. Yafei Jiang for discussion.
Author information
Authors and Affiliations
Contributions
Li J directed the research. Zhu C, Liang JX and Meng Y conducted the DFT calculations. Zhu C, Liang JX, Xu CQ and Meng Y analyzed the data. All the authors discussed the results and co-wrote the manuscript.
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
See supplementary material for the way of oxygen-terminated Ti2C, the different adsorption sites of metal single atoms on Os1/Ti2CS2, as well as the optimized geometry of Ir1/Ti2CS2. In addition, the optimized stable structures and the calculated relative energies of different molecule adsorption on Os1/Ti2CS2 SAC are also available in the online version of the paper.
Yang Meng is a PhD candidate at the School of Chemistry and Chemical Engineering, Guizhou University, under the supervision of Prof. Chun Zhu. Her research focuses on the theoretical investigations on single-atom catalysts.
Chun Zhu received his PhD degree from the College of Chemistry and Chemical Engineering, Xiamen University in 2013, under the supervision of Prof. Zexing Cao. He worked as a visiting scholar at the Department of Chemistry and the Department of Biochemistry and Molecular Biology, Michigan State University from 2017 to 2018. He was also a visiting scholar at the Department of Chemistry, Southern University of Science and Technology. He is now a professor at the School of Chemistry and Chemical Engineering, Guizhou University. His research interests focus on the theoretical chemistry, organometallic chemistry, and computational catalysis science.
Jun Li received his PhD degree from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences in 1992. He did postdoctoral research at the University of Siegen (Germany) and The Ohio State University (USA) from 1994 to 1997. He worked as a Research Scientist at The Ohio State University and a Senior Research Scientist and Chief Scientist at the Pacific Northwest National Laboratory (USA) from 1997 to 2009. He is now a full professor at Tsinghua University. His research involves theoretical chemistry, relativistic heavy-element chemistry, and computational catalysis science.
Rights and permissions
About this article
Cite this article
Meng, Y., Liang, JX., Zhu, C. et al. Theoretical studies of MXene-supported single-atom catalysts: Os1/Ti2CS2 for low-temperature CO oxidation. Sci. China Mater. 65, 1303–1312 (2022). https://doi.org/10.1007/s40843-021-1950-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1950-0