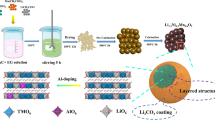
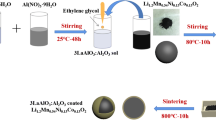
Abstract
Layered Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (0 ≤ x ≤ 0.08) cathode materials were successfully synthesized by a sol-gel method. X-ray diffraction and the refinement data indicate that all materials have typical α-NaFeO2 structure with R-3m space group, and the a-axis has almost no change, but there is a slight decrease in the c lattice parameter as well as the cell volume. Scanning electron microscopy and high resolution transmission electron microscopy prove that all the samples have uniform particle size of about 200–300 nm and smooth surface. The energy-dispersive X-ray spectroscopy mapping shows that aluminum has been homogeneously doped in the Li1.2Mn0.56Ni0.16Co0.08O2 cathode material. The cyclic voltammetry and electrochemical impedance spectroscopy reveal that appropriate Al-doping contributes to the reversible lithium-ion insertion and extraction, and then reduces the electrochemical polarization and charge transfer resistance. Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x = 0.05) shows the lowest charge transfer resistance and the highest lithium-ion diffusion coefficient among all the samples. The Li-rich electrodes with low-level Al doping shows a much higher discharge capacity than the pristine one, especially the Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x = 0.05) sample, which exhibits greater rate capacity and better fast charge-discharge performance than the other samples. Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x = 0.05) also exhibits higher discharge capacity than the pristine one at each cycle at 55°C. These results clearly indicate that the high rate capacity together with a good high rate cycling performance and high-temperature performance of the low-Co Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x=0.05) is a promising alternative to next-generation lithium-ion batteries.
摘要
本文采用溶胶凝胶法成功合成了层状Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (0≤x≤0.08)正极材料. XRD及其精细结果表明, 所有的材料均具有 典型的α-NaFeO2结构, 属于R-3m空间群. Al掺杂后的材料晶胞参数a值几乎不变, 但是c值和晶胞体积略微减小. SEM和HRTEM证明了所有 样品均具有200–300 nm的均一粒径和光滑的表面. EDS谱图说明Al已经成功地进入了Li1.2Mn0.56Ni0.16Co0.08O2正极材料的晶格. CV和EIS说 明适量的Al掺杂有利于锂离子的可逆脱嵌, 减小了材料的电化学极化和电荷转移电阻. 在所有样品中, Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x=0.05) 展示了最小的电荷转移电阻和最高的锂离子扩散系数. 电化学性能测试表明, 少量Al掺杂的富锂电极具有比纯样更高的放电容量, 特 别是Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x=0.05)样品具有比其他样品更高的倍率容量和更好的快速充放电性能. 55°C时, Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x=0.05)展示了比纯样更高的放电容量. 高的倍率容量、好的高倍率循环稳定性以及优秀的高温性能使得低钴Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (x=0.05)材料成为下一代锂离子电池颇具前景的选择.
Similar content being viewed by others
References
Li W, Zeng L, Wu Y, et al. Nanostructured electrode materials for lithium-ion and sodium-ion batteries via electrospinning. Sci China Mater, 2016, 59: 287–321
Zhang J, Yu A. Nanostructured transitionmetal oxides as advanced anodes for lithium-ion batteries. Sci Bull, 2015, 60: 823–838
Song L, Yang S, WeiW, et al. Hierarchical SnO2 nanoflowers assembled by atomic thickness nanosheets as anode material for lithium ion battery. Sci Bull, 2015, 60: 892–895
Wang L, Hu Z, Zhao K, et al. Hollow spherical LiNi0.5Mn1.5O4 built from polyhedra with high-rate performance via carbon nanotube modification. Sci China Mater, 2016, 59: 95–103
Wu C, Yuan L, Li Z, et al. High-performance lithium-selenium battery with Se/microporous carbon composite cathode and carbonate-based electrolyte. Sci China Mater, 2015, 58: 91–97
Sun X, Yan C, Chen Y, et al. Three-dimensionally “curved” NiO nanomembranes as ultrahigh rate capability anodes for Li-ion batteries with long cycle lifetimes. Adv Energy Mater, 2014, 4: 1300912
Massé RC, Uchaker E, Cao G. Beyond Li-ion: electrode materials for sodium- and magnesium-ion batteries. Sci China Mater, 2015, 58: 715–766
Liu X, SuQ, ZhangC, et al. Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode with an ionic conductive LiVO3 coating layer. ACS Sustain Chem Eng, 2016, 4: 255–263
Sun YY, Li F, Qiao QQ, et al. Surface modification of Li(Li0.17Ni0.2Co0.05Mn0.58)O2 with LiAlSiO4 fast ion conductor as cathode material for Li-ion batteries. Electrochim Acta, 2015, 176: 1464–1475
Nayak PK, Grinblat J, Levi E, et al. Effect of cycling conditions on the electrochemical performance of high capacity Li and Mn-rich cathodes for Li-ion batteries. J Power Sources, 2016, 318: 9–17
Zhuo H, Zhang Y, Wang D, et al. Insight into lithium-rich layered cathode materials Li[Li0.1Ni0.45Mn0.45]O2 in situ coated with graphene-like carbon. Electrochim Acta, 2014, 149: 42–48
Zhao E, Liu X, Hu Z, et al. Facile synthesis and enhanced electrochemical performances of Li2TiO3-coated lithium-rich layered Li1.13Ni0.30Mn0.57O2 cathode materials for lithium-ion batteries. J Power Sources, 2015, 294: 141–149
Xiang Y, Li J, Wu X, et al. Synthesis and electrochemical characterization of Mg-doped Li-richMn-based cathode material. Ceramics Int, 2016, 42: 8833–8838
Li L, Song BH, Chang YL, et al. Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. J Power Sources, 2015, 283: 162–170
Zhao Y, Xia M, Hu X, et al. Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.8O2 Li-rich cathode materials. Electrochim Acta, 2015, 174: 1167–1174
Huang Z, Wang Z, Jing Q, et al. Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathodematerial. Electrochim Acta, 2016, 192: 120–126
Oh P, Myeong S, Cho W, et al. Superior long-termenergy retention and volumetric energy density for Li-rich cathode materials. Nano Lett, 2014, 14: 5965–5972
Lee Y, Kim MG, Cho J. Layered Li0.88[Li0.18Co0.33Mn0.49]O2 nanowires for fast and high capacity Li-Ion storage material. Nano Lett, 2008, 8: 957–961
Yan J, Liu X, Li B. Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv, 2014, 4: 63268–63284
Toprakci O, Toprakci HAK, Li Y, et al. Synthesis and characterization of xLi2MnO3·(1−x)LiMn1/3Ni1/3Co1/3O2 composite cathode materials for rechargeable lithium-ion batteries. J Power Sources, 2013, 241: 522–528
Yi TF, Tao W, Chen B, et al. High-performance xLi2MnO3·(1−x)LiMn1/3Co1/3Ni1/3O2 (0.1≤x≤0.5) as cathode material for lithium-ion battery. Electrochim Acta, 2016, 188: 686–695
Ghanty C, Basu RN, Majumder SB. Electrochemical characteristics of xLi2MnO2-(1−x)Li(Mn0.375Ni0.375Co0.25)O2 (0.0≤x≤1.0) composite cathodes: effect of particle and Li2MnO2 domain size. Electrochim Acta, 2014, 132: 472–482
Zhang Q, Peng T, Zhan D, et al. Synthesis and electrochemical property of xLi2MnO2·(1−x)LiMnO2 composite cathode materials derived from partially reduced Li2MnO3. J Power Sources, 2014, 250: 40–49
He P, Yu H, Li D, et al. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J Mater Chem, 2012, 22: 3680
Ye D, Wang B, Chen Y, et al. Understanding the stepwise capacity increase of high energy low-Co Li-rich cathode materials for lithium ion batteries. J Mater Chem A, 2014, 2: 18767–18774
Huang X, Wang M, Che R. Modulating the Li+/Ni2+ replacement and electrochemical performance optimizing of layered lithiumrich Li1.2Ni0.2Mn0.6O2 by minor Co dopant. J Mater Chem A, 2014, 2: 9656–9665
Fan J, Li G, Luo D, et al. Hydrothermal-assisted synthesis of Li-rich layered oxide microspheres with high capacity and superior ratecapability as a cathode for lithium-ion batteries. Electrochim Acta, 2015, 173: 7–16
Yi TF, Mei J, Zhu YR. Key strategies for enhancing the cycling stability and rate capacity of LiNi0.5Mn1.5O4 as high-voltage cathode materials for high power lithium-ion batteries. J Power Sources, 2016, 316: 85–105
Yi T, Hu X, Gao K. Synthesis and physicochemical properties of LiAl0.05Mn1.95O4 cathode material by the ultrasonic-assisted sol-gel method. J Power Sources, 2006, 162: 636–643
Liu L, Xie F, Lyu J, et al. Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries. J Power Sources, 2016, 321: 11–35
Zhao R, Zhang S, Liu J, et al. A review of thermal performance improving methods of lithium ion battery: electrode modification and thermal management system. J Power Sources, 2015, 299: 557–577
Yang C, Zhang Q, Ding W, et al. Improving the electrochemical performance of layered lithium-rich cathode materials by fabricating a spinel outer layer with Ni3+. J Mater Chem A, 2015, 3: 7554–7559
Thackeray MM, Kang SH, Johnson CS, et al. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem, 2007, 17: 3112–3125
Chen Z, Dahn JR. Effect of a ZrO2 coating on the structure and electrochemistry of LixCoO2 when cycled to 4.5 V. Electrochem Solid-State Lett, 2002, 5: A213
Wang CC, Lin YC, Chou PH. Mitigation of layer to spinel conversion of a lithium-rich layered oxide cathode by substitution of Al in a lithium ion battery. RSC Adv, 2015, 5: 68919–68928
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A, 1976, 32: 751–767
Mohanty D, Sefat AS, Kalnaus S, et al. Investigating phase transformation in the Li1.2Co0.1Mn0.55Ni0.15O2 lithium-ion battery cath-ode during high-voltage hold (4.5 V) via magnetic, X-ray diffraction and electron microscopy studies. J Mater Chem A, 2013, 1: 6249–6261
Zheng Z, Wu ZG, Zhong YJ, et al. A further electrochemical investigation on solutions to high energetical power sources: isomerous compound 0.75Li1.2Ni0.2Mn0.6O2·0.25LiNi0.5Mn1.5O4. RSC Adv, 2015, 5: 37330–37339
Wang D, Zhao Y, Xu X, et al. Novel Li2MnO3 nanowire anode with internal Li-enrichment for use in a Li-ion battery. Nanoscale, 2014, 6: 8124–8129
Zhang X, Yin Y, Hu Y, et al. Zr-containing phosphate coating to enhance the electrochemical performances of Li-rich layer-structured Li[Li0.2Ni0.17Co0.07Mn0.56]O2. Electrochim Acta, 2016, 193: 96–103
Pang S, Wang Y, Chen T, et al. The effect of AlF3 modification on the physicochemical and electrochemical properties of Li-rich layered oxide. Ceramics Int, 2016, 42: 5397–5402
Yu FD, Wang ZB, Chen F, et al. Crystal structure andmulticomponent effects in Li1+x Mn2−x−y AlyO4 cathode materials for Li-ion batteries. J Power Sources, 2014, 262: 104–111
Liu H, Wen G, Bi S, et al. High rate cycling performance of nanosized Li4Ti5O12/graphene composites for lithium ion batteries. Electrochim Acta, 2016, 192: 38–44
Xiang X, LiW. Self-directed chemical synthesis of lithium-rich layered oxide Li[Li0.2Ni0.2Mn0.6]O2 with tightly interconnected particles as cathode of lithium ion batteries with improved rate capability. Electrochim Acta, 2014, 127: 259–265
Wu MS, Chiang PCJ, Lin JC. Electrochemical investigations on advanced lithium-ion batteries by three-electrode measurements. J Electrochem Soc, 2005, 152: A47–A52
Yi TF, Fang ZK, Xie Y, et al. Synthesis of LiNi0.5Mn1.5O4 cathode with excellent fast charge-discharge performance for lithium-ion battery. Electrochim Acta, 2014, 147: 250–256
Sun X, Si W, Liu X, et al. Multifunctional Ni/NiO hybrid nanomembranes as anode materials for high-rate Li-ion batteries. Nano Energy, 2014, 9: 168–175
Liu J, Manthiram A. Understanding the improved electrochemical performances of Fe-substituted 5 V spinel cathode LiMn1.5Ni0.5O4. J Phys Chem C, 2009, 113: 15073–15079
Duncan H, Duguay D, Abu-Lebdeh Y, et al. Study of the LiMn1.5Ni0.5O4/electrolyte interface at room temperature and 60°C. J Electrochem Soc, 2011, 158: A537
Lu D, Xu M, Zhou L, et al. Failure mechanism of graphite/LiNi0.5Mn1.5O4 cells at high voltage and elevated temperature. J Electrochemical Soc, 2013, 160: A3138–A3143
Chen J, Zhang H, Wang M, et al. Improving the electrochemical performance of high voltage spinel cathode at elevated temperature by a novel electrolyte additive. J Power Sources, 2016, 303: 41–48
Jafta CJ, Ozoemena KI, Mathe MK, et al. Synthesis, characterisation and electrochemical intercalation kinetics of nanostructured aluminium-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodematerial for lithium ion battery. Electrochim Acta, 2012, 85: 411–422
Author information
Authors and Affiliations
Corresponding author
Additional information
Ting-Feng Yi received his BE degree in chemical engineering and technology from Liaocheng University in 2001. He then obtained MSc degree in applied chemistry in 2004 and PhD degree in chemical engineering and technology from Harbin Institute of Technology in 2007. He joined Anhui University of Technology as an assistant professor of chemistry in 2007. He is currently the professor of Anhui University of Technology, China. His research interests include synthesis of electrochemical functional materials and their application in lithium-ion battery and lead-acid battery. For detail please see his researcher ID: http://www.researcherid.com/rid/F-4594-2012.
Electronic supplementary material
40843_2016_5097_MOESM1_ESM.pdf
Enhanced electrochemical performance of Li-rich low-Co Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (0≤x≤0.08) as cathode materials
Rights and permissions
About this article
Cite this article
Yi, TF., Han, X., Yang, SY. et al. Enhanced electrochemical performance of Li-rich low-Co Li1.2Mn0.56Ni0.16Co0.08−x Al x O2 (0≤x≤0.08) as cathode materials. Sci. China Mater. 59, 618–628 (2016). https://doi.org/10.1007/s40843-016-5097-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-016-5097-7