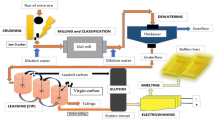
Abstract
Current industrial processes of tantalum (Ta) and niobium (Nb) extraction from primary resources rely in majority on hydrofluoric (HF) acid leaching, which is raising material handling and safety issues. In this work, a novel HF-free treatment route for a coltan ore, based on alkaline roasting, water-leaching, precipitation, and oxalic leaching is presented. A raw ore from Lulingu deposit (the Democratic Republic of the Congo) was subjected to alkaline roasting followed by water leaching to bring Ta and Nb into solution. The most influential operating parameters of the roasting were outlined using Taguchi orthogonal array L16 (44) method. The optimal roasting conditions determined as temperature (450 °C), roasting time (2 h), molar ratio KOH/(Ta,Nb)2O5 of 13.2 (e.g., mass ratio KOH to ore of about 2/1) and granulometric fraction (− 105 + 75 µm), enabled about 87% of Ta and 92% of Nb to be subsequently water leached. The resulting alkaline pregnant leach solution (PLS) was subjected to precipitation, followed by oxalic acid leaching of the formed precipitates. This step brought about 86% of Ta and 92% of Nb in solution confirming the efficiency of the method. Globally, an extraction efficiency of ca. 75% for Ta and 84% for Nb could be expected from the entire process. In addition, the obtained Ta–Nb leachate was deemed suitable to downstream solvent extraction in view yielding high-purity Ta2O5 and Nb2O5 compounds.
Graphical Abstract













Similar content being viewed by others
References
European Commission Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 (2017) List of critical raw materials for the EU. European Commission Brussels. https://ec.europa.eu/transparency/regdoc/rep/1/2017/EN/COM-2017-490-F1-EN-MAINPART-1.PDF. Accessed 18 May 2021
Schulz JF, Piatak NM, Papp JF (2017) Niobium and tantalum-critical mineral resources of the United States—economic and environmental geology and prospects for future. US Geol Surv 1802:1–34
Lessard JD, Shekhter LN, Gribbin DG, Blagoveshchensky Y, McHugh LF (2015) A new technology platform for the production of electronic grade tantalum nanopowders from tantalum scrap sources. Int J Refract Met Hard Mater 48:408–413. https://doi.org/10.1016/j.ijrmhm.2014.09.027
Habinshuti JB, Munganyinka JP, Adetunji AR, Mishra B, Ofori-Sarpong G, Komadja GC, Gbetoglo C, Himanshu T, Mukiza J, Onwualu AP (2021) Mineralogical and physical studies of low-grade tantalum-tin ores from selected areas of Rwanda. Results Eng 11:100248. https://doi.org/10.1016/j.rineng.2021.100248
Shikika A, Sethurajan M, Muvundja F, Mugumaoderha MC, St G (2020) A review on extractive metallurgy of tantalum and niobium. Hydrometallurgy 198:1–13. https://doi.org/10.1016/j.hydromet.2020.105496
Shikika A, Zabene F, Muvundja F, Mugumaoderha MC, Colaux JL, Aatach M, St G (2021) Extraction of Ta and Nb from a coltan bearing ore by means of ammonium bifluoride fluorination and sulfuric acid leaching. Minerals 11:1–17. https://doi.org/10.3390/min11121392
Yang XL, Wang XH, Wei C, Zheng SL, Sun Q (2013) Decomposition of niobium ore by sodium hydroxide fusion method. Metall Mater Trans B 44(1):45–52. https://doi.org/10.1007/s11663-012-9766-8
Yang X, Zhang J, Fang X, Qiu T (2014) Kinetics of pressure leaching of niobium ore by sulfuric acid. Int J Refract Met Hard Mater 45:218–222. https://doi.org/10.1016/j.ijrmhm.2014.04.001
Brocchi E (1983) Reduction chlorination reactions of niobium and tantalum oxide containing materials. PhD thesis, University of London, London
Gaballah I, Allain E, Djona M (1997) Extraction of tantalum and niobium from tin slags by chlorination and carbochlorination. Metall Mater Trans B 28(3):359–369. https://doi.org/10.1007/s11663-997-0102-7
Brocchi E, Moura FJ (2008) Chlorination methods applied to recover refractory metals from tin slags. Miner Eng 21(2):150–156. https://doi.org/10.1016/j.mineng.2007.08.011
Rodriguez M, Quiroga O, Ruiz M (2007) Kinetic study of ferrocolumbite dissolution in hydrofluoric acid medium. Hydrometallurgy 85(2–4):87–94. https://doi.org/10.1016/j.hydromet.2006.07.005
Masloboeva SM, Arutyunyan LG, Palatnikov MN, Manukovskaya DV (2021) Separation and purification of tantalum from plumbomicrolite of amazonite deposit in Kola Peninsula by acid leaching and solvent extraction. J Cent South Univ 28(1):72–88. https://doi.org/10.1007/s11771-021-4587-z
Zhou HM, Yi DQ, Zhang Y, Zheng SL (2005) The dissolution behavior of Nb2O5, Ta2O5 and their mixture in highly concentrated KOH solution. Hydrometallurgy 80(1–2):126–131. https://doi.org/10.1016/j.hydromet.2005.07.010
Wang X, Zheng S, Xu H, Zhang Y (2009) Leaching of niobium and tantalum from a low-grade ore using a KOH roast-water leach system. Hydrometallurgy 98(3–4):219–223. https://doi.org/10.1016/j.hydromet.2009.05.002
Cardarelli F (2000) Less common nonferrous metals. Mater Handb. https://doi.org/10.1007/978-1-4471-3648-4_3
Waters T, Wedd AG, Ziolek M, Nowak I (2004) Niobium and Tantalum. Compr Coord Chem II 4:219–228. https://doi.org/10.1016/B0-08-043748-6/03031-0
Maiorov VG et al (2012) On potassium hexaniobates released in the course of preparation of concentrated niobium(V) alkaline solution. Russ J Appl Chem 85(12):1820–1826. https://doi.org/10.1134/S1070427212120051
Nikolaev AI, Maiorov VG, Kopkov VK, Zalkind OA, Kadyrova GI (2012) Preparation of concentrated alkali solutions of niobium. Theor Found Chem Eng 46(5):563–566. https://doi.org/10.1134/S0040579512050065
Habinshuti JB, Munganyinka JP, Adetunji R, Brajendra M, Tanvar H, Mukiza J, Ofori-Sarpong G, Onwualu AP (2022) Caustic potash assisted roasting of the Nigerian ferro-columbite concentrate and guanidine carbonate-induced precipitation: a novel technique for extraction of Nb–Ta mixed-oxides. Results Eng 14(4):100415. https://doi.org/10.1016/j.rineng.2022.100415
Li SC, Kang CS, Kim SC, Kim CJ, Kang CJ (2019) The extraction of Ta, Nb and rare earths from fergusonite by using KOH sub-molten salt leaching. Hydrometallurgy 201(6):105358. https://doi.org/10.1016/j.hydromet.2020.105358
El Hazek MN, Mohamed NH, Gabr AA (2019) Potash breakdown of poly-mineralized niobium-tantalum-lanthanides ore material. Am J Anal Chem 10(3):103–111. https://doi.org/10.4236/ajac.2019.103009
Sun L, Yu H, Meng F, Qi T, Wang L, Peng Y (2021) Recovery of niobium and titanium from ilmenorutile by NaOH roasting-H2SO4 leaching process. J Mater Res Technol 15:2575–2583. https://doi.org/10.1016/j.jmrt.2021.09.091
Sanchez-Segado A, Ruzaidi A, Zhang Y, Jha A (2015) Characterization of physico-chemical changes during the alkali roasting of niobium and tantalum oxides. In: Drying, roasting, and calcining of minerals. Springer, Cham, pp 51–58
Deblonde GJP et al (2016) Selective recovery of niobium and tantalum from low-grade concentrates using a simple and fluoride-free process. Sep Purif Technol 162:180–187. https://doi.org/10.1016/j.seppur.2016.02.025
Zhou KG, Tokuda M (2000) Solvent extraction of niobium from alkali solution by methyltrioctylammonium chloride. J Cent South Univ Technol (English Ed) 7(4):176–177. https://doi.org/10.1007/s11771-000-0047-3
Deblonde GJP, Chagnes A, Roux MA, Weigel V, Cote G (2016) Extraction of Nb(v) by quaternary ammonium-based solvents: toward organic hexaniobate systems. Dalton Trans 45(48):19351–19360. https://doi.org/10.1039/C6DT03873E
Eramet, Deblonde G, Roux M-A, Weigel V, Chagnes A, Cote G (2017) Hydrometallurgical method for separating and purifying tantalum and niobium. A Patent, WO 2017/085404 Al
Deblonde GJP, Bengio D, Beltrami D, Bélair S, Cote G, Chagnes A (2019) A fluoride-free liquid-liquid extraction process for the recovery and separation of niobium and tantalum from alkaline leach solutions. Sep Purif Technol 215:634–643. https://doi.org/10.1016/j.seppur.2019.01.052
Deblonde GJP, Chagnes A, Weigel V, Cote G (2016) Direct precipitation of niobium and tantalum from alkaline solutions using calcium-bearing reagents. Hydrometallurgy 165:345–350. https://doi.org/10.1016/j.hydromet.2015.12.009
Chang KI, Hong SI (2008) Effect of sulphur on the strengthening of a Zr–Nb alloy. J Nucl Mater 373(1–3):16–21. https://doi.org/10.1016/j.jnucmat.2007.04.045
Bass I (2007) Six Sigma Statistics with Excel and Minitab. The McGraw, New York, p 2007
Wang XH, Zheng SL, Bin XuH, Zhang Y (2010) Dissolution behaviors of Ta2O5, Nb2O5 and their mixture in KOH and H2O system. Trans Nonferrous Met Soc China (English Ed) 20(10):2006–2011. https://doi.org/10.1016/S1003-6326(09)60409-X
Shikika A, Muvundja F, Mugumaoderha MC, Gaydardzhiev S (2021) Extraction of Nb and Ta from a coltan ore from South Kivu in the DRC by alkaline roasting—thermodynamic and kinetic aspects. Miner Eng 163(3):1–11. https://doi.org/10.1016/j.mineng.2020.106751
Berhe GG, Alberto VDR, Tadesse B, Yimam A, Woldetinsae G (2018) Decomposition of the Kenticha mangano-tantalite ore by HF/H2SO4 and KOH fusion. Physicochem Probl Miner Process 54(2):406–414. https://doi.org/10.5277/ppmp1840
Escudero-Castejon L, Sanchez-Segado S, Parirenyatwa S, Jha A (2016) Formation of chromium-containing molten salt phase during roasting of chromite ore with sodium and potassium hydroxides. J Manuf Sci Prod 16(4):215–225. https://doi.org/10.1515/jmsp-2016-0023
Nkulu G, Gaydardzhiev S, Mwema E (2013) Statistical analysis of bioleaching copper, cobalt, and nickel from polymetalic concentrate originating from Kamoya deposit in the Democratic Republic of Congo. Miner Eng 48:77–85. https://doi.org/10.1016/j.mineng.2012.10.007
Deblonde GJP, Moncomble A, Cote G, Bélair S, Chagnes A (2015) Experimental and computational exploration of the UV–visible properties of hexaniobate and hexatantalate ions. RSC Adv 5(10):7619–7627. https://doi.org/10.1039/c4ra14866e
Ihli J et al (2015) Precipitation of amorphous calcium oxalate in aqueous solution. Chem Mater 27(11):3999–4007. https://doi.org/10.1021/acs.chemmater.5b01642
Talerico C, Ochs M, Giffaut E (2004) Solubility of niobium(V) under cementitious conditions: importance of Ca-niobate. Mater Res Soc Symp Proc 824:414–419. https://doi.org/10.1557/PROC-824-CC8.31
Jehng J-M, Wachs IE (1991) Niobium oxide solution chemistry. J Raman Spectrosc 22(2):83–89. https://doi.org/10.1002/jrs.1250220207
Zhu Z, Cheng CY (2011) Solvent extraction technology for the separation and purification of niobium and tantalum: a review. Hydrometallurgy 107(1–2):1–12. https://doi.org/10.1016/j.hydromet.2010.12.015
Wu B, Shang H, Wen JK (2015) Sulfuric acid leaching of low-grade refractory tantalum–niobium and associated rare earths minerals in Panxi area of China. Rare Met 34(3):202–206. https://doi.org/10.1007/s12598-014-0436-7
Djordjević C, Goričan H, Tan SL (1966) Solvent extraction of niobium and tantalum: extraction mechanism in oxalic solutions with long chain tertiary amines. J Less-Common Met 11(5):342–350. https://doi.org/10.1016/0022-5088(66)90066-X
Yang X, Zhang J, Fang X (2015) Extraction kinetics of niobium by tertiary amine N235 using Lewis cell. Hydrometallurgy 151:56–61. https://doi.org/10.1016/j.hydromet.2014.11.007
Deblonde GJP, Bengio D, Beltrami D, Bélair S, Cote G, Chagnes A (2019) Niobium and tantalum processing in oxalic-nitric media: Nb2O5·nH2O and Ta2O5·nH2O precipitation with oxalates and nitrates recycling. Sep Purif Technol 226(5):209–217. https://doi.org/10.1016/j.seppur.2019.05.087
Sun L et al (2021) A novel method for the separation of niobium and titanium from sulfuric acid-oxalate solutions using N235 and MIBK. Hydrometallurgy 205(1):105748. https://doi.org/10.1016/j.hydromet.2021.105748
AbdelWahab GM, Abdellah WM, Yousif AM, Mubark AE (2019) Preparation of pure Nb2O5 from Gabal El-Faliq Pegmatite, South Eastern Desert, Egypt. Min Metall Explor. https://doi.org/10.1007/s42461-019-00136-1
He BJ, Dajuan ZZXZN, Li (1997) Hydrometallurgical extraction of tantalum and niobium in China. Tantalum-niobium International Study Center (TIC), Lasne, pp 1–8
Polyakov EG, Polyakova LP (2003) Current trends in the production of tantalum and niobium. Metallurgist 47:33–41. https://doi.org/10.1023/A:1023874211543
Acknowledgements
This work was supported by the ARES Academy (Belgium) within the PRD 2019 ARES-CCD project. Prof. Fr. Hatert, Dr. H. Bouzahzah, and Dr. J.L. Colaux are gratefully acknowledged for their support with the mineralogical inspection. A. S. and F. Z. would like to thank the CEGEMI team led by Prof. Marie Rose Bashwira for their assistance during the coltan sampling campaign.
Author information
Authors and Affiliations
Contributions
AS: experiments execution, data compilation, and writing the initial draft. FZ: ore sampling, and materials preparation. FAM: background, data supply, and conceptualization. MM: background, data supply conceptualization, and additional samples preparation for elemental analysis. MA: experimental set-up, results discussion. SG: experiments planning, formulation of research aims, and paper concept as well as final paper polishing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript, and there is no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors certify that the submission is original work and is not under review at any other publication.
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shikika, A., Zabene, F., Muvundja, F.A. et al. Efficient Extraction of Ta and Nb from a Coltan Ore Through Alkaline Roasting, Water Leaching, Precipitation, and Oxalic Acid Leaching. J. Sustain. Metall. 8, 1932–1947 (2022). https://doi.org/10.1007/s40831-022-00621-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00621-w