Abstract
Purpose of Review
The goal is to examine the link between circadian rhythms and pain, which may shed further light on improving pain management strategies and preventing the development and/or worsening of chronic pain.
Recent Findings
In part I, we provide evidence that the rhythmicity of pain may be regulated by the central circadian clock. We also found that rhythmicity in pain widely differs across pain conditions. In part II, we provide indirect evidence that circadian rhythm disruptions (e.g., night-shift work) and improvement of circadian synchronization (e.g., light therapy) are associated with pain-related experiences.
Summary
Investigating the link between circadian rhythms and pain may inform enhancing precision pain medicine and assay sensitivity of clinical trials, as well as development of more effective chronic pain treatment and prevention programs. More rigorous laboratory studies that evaluate endogenous circadian pain rhythmicity and through what mechanisms disrupted circadian rhythms impact pain processing are needed.

Similar content being viewed by others
Notes
Static pain measures evaluate the sensory thresholds and tolerance to a single stimulus (e.g., pain threshold and tolerance), whereas dynamic pain measures can assess both the ascending pain facilitation and descending pain modulation of nociceptive signals (e.g., temporal summation and conditioned pain modulation) by inducing a number of painful stimuli [98].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160:19–27.
Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States Adults: results of an internet-based survey. J Pain. 2010;11:1230–9.
Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–24.
Aschoff J. Circadian rhythms in man. Science. 1965;80(148):1427–32.
Foster RG. Sleep, circadian rhythms and health. Interface Focus. 2020;10:20190098.
Ancoli-Israel S, Liu L, Natarajan L, Rissling M, Neikrug AB, Youngstedt SD, Mills PJ, Sadler GR, Dimsdale JE, Parker BA. Reductions in sleep quality and circadian activity rhythmicity predict longitudinal changes in objective and subjective cognitive functioning in women treated for breast cancer. Support Care Cancer. 2022;30:3187–200.
Bumgarner JR, Walker II WH, Nelson RJ (2021) Circadian rhythms and pain. Neurosci Biobehav Rev 129:296–306. An important review paper focusing on circadian rhythms and pain.
Warfield AE, Prather JF, Todd WD. Systems and circuits linking chronic pain and circadian rhythms. Front Neurosci. 2021;15:1–22.
Chu Y, He H, Liu Q, Jia S, Fan W, Huang F (2022) The circadian clocks, oscillations of pain-related mediators, and pain. Cell Mol Neurobiol 1–13. A review paper that provides overview of cellular and molecular mechanisms underlying pain and circadian rhythms.
Mun CJ, Suk HW, Davis MC, Karoly P, Finan P, Tennen H, Jensen MP. Investigating intra-individual pain variability: methods, applications, issues, and directions. Pain. 2019;160:2415–29.
Mun CJ, Weaver KR, Hunt CA, Owens MA, Phillips J, Lerman SF, Buenaver LF, Colloca L, Tennen H, Haythornthwaite JA. Pain expectancy and positive affect mediate the day-to-day association between objectively measured sleep and pain severity among women with temporomandibular disorder. J Pain. 2022;23:669–79.
Conner TS, Tennen H, Zautra AJ, Affleck G, Armeli S, Fifield J. Coping with rheumatoid arthritis pain in daily life: within-person analyses reveal hidden vulnerability for the formerly depressed. Pain. 2006;126:198–209.
Mun CJ, Thummala K, Davis MC, Karoly P, Tennen H, Zautra AJ. Predictors and social consequences of daily pain expectancy among adults with chronic pain. Pain. 2017;158:1224–33.
Moscou-Jackson G, Finan PH, Campbell CM, Smyth JM, Haythornthwaite JA. The effect of sleep continuity on pain in adults with sickle cell disease. J Pain. 2015;16:587–93.
Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: a daily diary study of people with reflex sympathetic dystrophy syndrome. J Consult Clin Psychol. 1999;67:776.
Mun CJ, Davis MC, Campbell CM, Finan PH, Tennen H. Linking nonrestorative sleep and activity interference through pain catastrophizing and pain severity: An intraday process model among individuals with fibromyalgia. J Pain. 2020;21:546–56.
Hagenauer MH, Crodelle JA, Piltz SH, Toporikova N, Ferguson P, Booth V. The modulation of pain by circadian and sleep-dependent processes: a review of the experimental evidence. Women Math Biol. 2017;1–21.
Enck P, Kaiser C, Felber M, Riepl RL, Klauser A, Klosterhalfen S, Otto B. Circadian variation of rectal sensitivity and gastrointestinal peptides in healthy volunteers. Neurogastroenterol Motil. 2009;21:52–8.
Aviram J, Shochat T, Pud D. Pain perception in healthy young men is modified by time-of-day and is modality dependent. Pain Med. 2015;16:1137–44.
Burish MJ, Chen Z, Yoo S. Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiol. 2019;225: e13161.
Cutolo M. Circadian rhythms and rheumatoid arthritis. Jt Bone Spine. 2019;86:327–33.
Boscariol R, Gilron I, Orr E. Chronobiological characteristics of postoperative pain: diurnal variation of both static and dynamic pain and effects of analgesic therapy. Can J Anesth. 2007;54:696–704.
Bruguerolle B, Labrecque G. Rhythmic pattern in pain and their chronotherapy. Adv Drug Deliv Rev. 2007;59:883–95.
Barloese M, Lund N, Petersen A, Rasmussen M, Jennum P, Jensen R. Sleep and chronobiology in cluster headache. Cephalalgia. 2015;35:969–78.
Steinberg A, Fourier C, Ran C, Waldenlind E, Sjöstrand C, Belin AC. Cluster headache–clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia. 2018;38:1286–95.
Burish MJ, Chen Z, Yoo S-H. Cluster headache is in part a disorder of the circadian system. JAMA Neurol. 2018;75:783–4.
Fox AW, Davis RL. Migraine chronobiology. J Head Face. Pain. 1998;38:436–41.
Alstadhaug K, Salvesen R, Bekkelund S. 24-Hour distribution of migraine attacks. J Head Face Pain. 2008;48:95–100.
Hu S, Gilron I, Singh M, Bhatia A. A scoping review of the diurnal variation in the intensity of neuropathic pain. Pain Med. 2022;23:991–1005.
Daguet I, Raverot V, Bouhassira D, Gronfier C (2020) Circadian rhythmicity of pain sensitivity in humans. Brain. This is a paper that examined endogenous circadian rhythmicity of pain sensitivity for the first time.
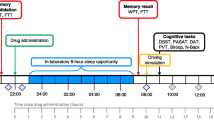
Duffy JF, Dijk D-J. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13.
Koorengevel KM, Beersma DGM, Den Boer JA, Van Den Hoofdakker RH. A forced desynchrony study of circadian pacemaker characteristics in seasonal affective disorder. J Biol Rhythms. 2002;17:463–75.
Kline CE, Durstine JL, Davis JM, Moore TA, Devlin TM, Youngstedt SD. Circadian rhythms of psychomotor vigilance, mood, and sleepiness in the ultra-short sleep/wake protocol. Chronobiol Int. 2010;27:161–80.
Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic non-cancer pain: the role of opioid prescription. Clin J Pain. 2014;30:557.
Glaser AM, Reyes-Vázquez C, Prieto-Gómez B, Burau K, Dafny N. Morphine administration and abrupt cessation alter the behavioral diurnal activity pattern. Pharmacol Biochem Behav. 2012;101:544–52.
Buttgereit F, Smolen JS, Coogan AN, Cajochen C. Clocking in chronobiology in rheumatoid arthritis. Nat Rev Rheumatol. 2015;11:349–56.
Junker U, Wirz S. Chronobiology: influence of circadian rhythms on the therapy of severe pain. J Oncol Pharm Pract. 2010;16:81–7.
Pöllmann L. Circadian variation of potency of placebo as analgesic. Funct Neurol. 1987;2:99–103.
Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:293–9.
Walker WH, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10:1–13.
Ebisawa T. Circadian rhythms in the CNS and peripheral clock disorders: human sleep disorders and clock genes. J Pharmacol Sci. 2007;103:150–4.
Lamont EW, James FO, Boivin DB, Cermakian N. From circadian clock gene expression to pathologies. Sleep Med. 2007;8:547–56.
Farhud D, Aryan Z. Circadian rhythm, lifestyle and health: a narrative review. Iran J Public Health. 2018;47:1068.
Karatsoreos IN. Effects of circadian disruption on mental and physical health. Curr Neurol Neurosci Rep. 2012;12:218–25.
Hannemann J, Laing A, Middleton B, Cridland J, Staels B, Marx N, Grant PJ, Federici M, Stenberg T, Skene DJ. Light therapy improves diurnal blood pressure control in night shift workers via reduction of catecholamines: the EuRhythDia study. J Hypertens. 2021;39:1678–88.
Xiao P, Ding S, Duan Y, Li L, Zhou Y, Luo X, Xie J, Cheng ASK. Effect of light therapy on cancer-related fatigue: a systematic review and meta-analysis. J Pain Symptom Manage. 2021;63:e188–202.
Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J Affect Disord. 2016;198:64–71.
Youngstedt SD, Kline CE, Reynolds AM, Crowley SK, Burch JB, Khan N, Han S. Bright light treatment of combat-related PTSD: a randomized controlled trial. Mil Med. 2021;187:e435–44.
Rissling M, Liu L, Youngstedt SD, Trofimenko V, Natarajan L, Neikrug AB, Jeste N, Parker BA, Ancoli-Israel S. Preventing sleep disruption with bright light therapy during chemotherapy for breast cancer: a phase II randomized controlled trial. Front Neurosci. 2022;16:815872.
Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. Chronotype and social jetlag: a (self-) critical review. Biology. 2019;8:54.
Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int. 2012;29:1153–75.
Au J, Reece J. The relationship between chronotype and depressive symptoms: a meta-analysis. J Affect Disord. 2017;218:93–104.
Kivelä L, Papadopoulos MR, Antypa N. Chronotype and psychiatric disorders. Curr Sleep Med Rep. 2018;4:94–103.
Jankowski KS. Morning types are less sensitive to pain than evening types all day long. Eur J Pain. 2013;17:1068–73.
Heikkala E, Oura P, Korpela T, Karppinen J, Paananen M (2022) Chronotypes and disabling musculoskeletal pain: a Finnish birth cohort study. Eur J Pain 26:1069–1078. This is a large cohort study that examines the association between chronotypes and pain.
Heikkala E, Paananen M, Merikanto I, Karppinen J, Oura P. Eveningness intensifies the association between musculoskeletal pain and health-related quality of life. Pain. Advance online publication. 2022.
Kim S, Park W-J, Cho S, Lim D-Y, Yoo Y, Kim H, Kang W, Kang KW, Moon J-D. The relationship between chronotypes and musculoskeletal problems in male automobile manufacturing workers. Ann Occup Environ Med. 2021;33:1–13.
Kantermann T, Theadom A, Roenneberg T, Cropley M. Fibromyalgia syndrome and chronotype: late chronotypes are more affected. J Biol Rhythms. 2012;27:176–9.
Türkoğlu G, Selvi Y. The relationship between chronotype, sleep disturbance, severity of fibromyalgia, and quality of life in patients with fibromyalgia. Chronobiol Int. 2020;37:68–81.
Chakradeo PS, Keshavarzian A, Singh S, Dera AE, Esteban JPG, Lee AA, Burgess HJ, Fogg L, Swanson GR. Chronotype, social jet lag, sleep debt and food timing in inflammatory bowel disease. Sleep Med. 2018;52:188–95.
Viticchi G, Falsetti L, Paolucci M, Altamura C, Buratti L, Salvemini S, Brunelli N, Bartolini M, Vernieri F, Silvestrini M. Influence of chronotype on migraine characteristics. Neurol Sci. 2019;40:1841–8.
Im H-J, Baek S-H, Yun C-H, Chu MK. Time preference of headache attack and chronotype in migraine and tension-type headache. Chronobiol Int. 2019;36:1528–36.
Van Oosterhout WPJ, Van Someren EJW, Schoonman GG, Louter MA, Lammers GJ, Ferrari MD, Terwindt GM. Chronotypes and circadian timing in migraine. Cephalalgia. 2018;38:617–25.
Bumgarner JR, Walker II WH, Liu JA, Walton JC, Nelson RJ (2020) Dim light at night exposure induces cold hyperalgesia and mechanical allodynia in male mice. Neuroscience 434:111–119. This is a well-controlled pre-clinical study suggesting the impact of circadian disruptions on pain.
Xu F, Zhao X, Liu H, Shao X, Chu S, Gong X, Ma Z, Gu X. Misaligned feeding may aggravate pain by disruption of sleep–awake rhythm. Anesth Analg. 2018;127:255–62.
Das V, Kc R, Li X, Varma D, Qiu S, Kroin JS, Forsyth CB, Keshavarzian A, van Wijnen AJ, Park TJ. Pharmacological targeting of the mammalian clock reveals a novel analgesic for osteoarthritis-induced pain. Gene. 2018;655:1–12.
Carvalho F, Pedrazzoli M, Gasparin A, Dos Santos F, Zortea M, Souza A, Torres I da SL, Fregni F, Caumo W (2019) PER3 variable number tandem repeat (VNTR) polymorphism modulates the circadian variation of the descending pain modulatory system in healthy subjects. Sci Rep 9:1–11. This is the first study that shows that a clock gene polymorphism is associated with a deficit in top-down pain inhibition.
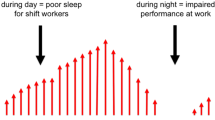
Brown JP, Martin D, Nagaria Z, Verceles AC, Jobe SL, Wickwire EM. Mental health consequences of shift work: an updated review. Curr Psychiatry Rep. 2020;22:1–7.
Itani O, Kaneita Y. The association between shift work and health: a review. Sleep Biol Rhythms. 2016;14:231–9.
Pieh C, Jank R, Waiß C, Pfeifer C, Probst T, Lahmann C, Oberndorfer S. Night-shift work increases cold pain perception. Sleep Med. 2018;45:74–9.
Takahashi M, Matsudaira K, Shimazu A. Disabling low back pain associated with night shift duration: sleep problems as a potentiator. Am J Ind Med. 2015;58:1300–10.
Eriksen W, Bruusgaard D, Knardahl S. Work factors as predictors of intense or disabling low back pain; a prospective study of nurses’ aides. Occup Environ Med. 2004;61:398–404.
Zhao I, Bogossian F, Turner C. The effects of shift work and interaction between shift work and overweight/obesity on low back pain in nurses: Results from a longitudinal study. J Occup Environ Med. 2012;54:820–5.
Waage S, Moen BE, Pallesen S, Eriksen HR, Ursin H, Åkerstedt T, Bjorvatn B. Shift work disorder among oil rig workers in the North Sea. Sleep. 2009;32:558–65.
Moreno CRC, Lowden A, Vasconcelos S, Marqueze EC. Musculoskeletal pain and insomnia among workers with different occupations and working hours. Chronobiol Int. 2016;33:749–53.
Matre D, Christensen JO, Mork PJ, Ferreira P, Sand T, Nilsen KB (2021) Shift work, inflammation and musculoskeletal pain—the HUNT study. Occup Med 71:422–427. A large cross-sectional study that shows the potential role of shift work on pain and inflammation.
Katsifaraki M, Nilsen KB, Christensen JO, Wærsted M, Knardahl S, Bjorvatn B, Härmä M, Matre D. Sleep duration mediates abdominal and lower-extremity pain after night work in nurses. Int Arch Occup Environ Health. 2019;92:415–22.
Christensen JO, Nilsen KB, Hopstock LA, Steingrímsdóttir ÓA, Nielsen CS, Zwart JA, Matre D. Shift work, low-grade inflammation, and chronic pain: a 7-year prospective study. Int Arch Occup Environ Health. 2021;94:1013–22.
Pfeffer M, Korf H-W, Wicht H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen Comp Endocrinol. 2018;258:215–21.
Danilov A, Kurganova J. Melatonin in chronic pain syndromes. Pain Ther. 2016;5:1–17.
Oh SN, Myung S-K, Jho HJ. Analgesic efficacy of melatonin: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Clin Med. 2020;9:1553.
Wang Z, Li Y, Lin D, Ma J. Effect of melatonin on postoperative pain and perioperative opioid use: a meta-analysis and trial sequential analysis. Pain Pract. 2021;21:190–203.
Zhu C, Xu Y, Duan Y, Li W, Zhang L, Huang Y, Zhao W, Wang Y, Li J, Feng T. Exogenous melatonin in the treatment of pain: a systematic review and meta-analysis. Oncotarget. 2017;8:100582.
Xie S, Fan W, He H, Huang F. Role of melatonin in the regulation of pain. J Pain Res. 2020;13:331.
Burgess HJ, Rizvydeen M, Kimura M, Pollack MH, Hobfoll SE, Rajan KB, Burns JW. An open trial of morning bright light treatment among US military veterans with chronic low back pain: A pilot study. Pain Med. 2019;20:770–8.
Burgess HJ, Park M, Ong JC, Shakoor N, Williams DA, Burns J (2017) Morning versus evening bright light treatment at home to improve function and pain sensitivity for women with fibromyalgia: a pilot study. Pain Med 18:116–123. One of the few studies that demonstrates a chronobiological intervention (i.e., bring light therapy) can improve chronic pain.
Leichtfried V, MatteucciGothe R, Kantner-Rumplmair W, Mair-Raggautz M, Bartenbach C, Guggenbichler H, Gehmacher D, Jonas L, Aigner M, Winkler D. Short-term effects of bright light therapy in adults with chronic nonspecific back pain: a randomized controlled trial. Pain Med. 2014;15:2003–12.
Martin LF, Patwardhan AM, Jain SV, Salloum MM, Freeman J, Khanna R, Gannala P, Goel V, Jones-MacFarland FN, Killgore WDS. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: a preliminary one-way cross-over clinical trial. Cephalalgia. 2021;41:135–47.
Martin L, Porreca F, Mata EI, Salloum M, Goel V, Gunnala P, Killgore WDS, Jain S, Jones-MacFarland FN, Khanna R. Green light exposure improves pain and quality of life in fibromyalgia patients: a preliminary one-way crossover clinical trial. Pain Med. 2021;22:118–30.
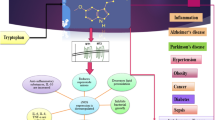
Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Res Rev. 2009;61:281–306.
Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–52.
Sivertsen B, Lallukka T, Petrie KJ, Steingrímsdóttir ÓA, Stubhaug A, Nielsen CS. Sleep and pain sensitivity in adults. Pain. 2015;156:1433–9.
Benarroch EE. Endogenous opioid systems: current concepts and clinical correlations. Neurology. 2012;79:807–14.
Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–805.
Xu H, Huang L, Zhao J, Chen S, Liu J, Li G. The circadian clock and inflammation: a new insight. Clin Chim Acta. 2021;512:12–7.
Matsuda M, Huh Y, Ji R-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33:131–9.
Zhang J-M, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45:27.
Mackey IG, Dixon EA, Johnson K, Kong J-T. Dynamic quantitative sensory testing to characterize central pain processing. JoVE. 2017;120:e54452.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Conflicts of Interests
Dr. Burgess reports personal fees from Natrol, LLC, Moving Mindz, Pty Ltd, and F. Hoffmann-La Roche Ltd outside the submitted work. The rest of the authors declare that they have no competing interests. We would also like to note that Fig. 1 represents the original image file from the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sleep and Pain
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mun, C.J., Burgess, H.J., Sears, D.D. et al. Circadian Rhythm and Pain: a Review of Current Research and Future Implications. Curr Sleep Medicine Rep 8, 114–123 (2022). https://doi.org/10.1007/s40675-022-00228-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-022-00228-3