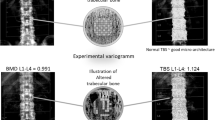
Abstract
Purpose
Bone is one of the main targets of hormones and endocrine diseases are frequent causes of secondary osteoporosis and fractures in real-world clinical practice. However, diagnosis of skeletal fragility and prediction of fractures in this setting could be a challenge, since the skeletal alterations induced by endocrine disorders are not generally captured by dual-energy X-ray absorptiometry (DXA) measurement of bone mineral density (BMD), that is the gold standard for diagnosis of osteoporosis in the general population. The aim of this paper is to review the existing evidence related to bone quality features in endocrine diseases, proposing assessment with new techniques in the future.
Methods
A comprehensive search within electronic databases was performed to collect reports of bone quality in primary hyperparathyroidism, hypoparathyroidism, hyperthyroidism, hypercortisolism, growth hormone deficiency, acromegaly, male hypogonadism and diabetes mellitus.
Results
Using invasive and non-invasive techniques, such as high-resolution peripheral quantitative computed tomography or DXA measurement of trabecular bone score (TBS), several studies consistently reported altered bone quality as predominant determinant of fragility fractures in subjects affected by chronic endocrine disorders.
Conclusions
Assessment of skeletal fragility in endocrine diseases might take advantage from the use of techniques to detect perturbation in bone architecture with the aim of best identifying patients at high risk of fractures.




Similar content being viewed by others
Data availability
Not applied.
References
Borgstrom F, Karlsson L, Ortsater G, Norton N, Halbout P, Cooper C, Lorentzon M, McCloskey EV, Harvey NC, Javaid MK, Kanis JA, International Osteoporosis F (2020) Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 15(1):59. https://doi.org/10.1007/s11657-020-0706-y
Curtis EM, Dennison EM, Cooper C, Harvey NC (2022) Osteoporosis in 2022: care gaps to screening and personalised medicine. Best Pract Res Clin Rheumatol 36(3):101754. https://doi.org/10.1016/j.berh.2022.101754
Eller-Vainicher C, Falchetti A, Gennari L, Cairoli E, Bertoldo F, Vescini F, Scillitani A, Chiodini I (2019) Diagnosis of endocrine disease: EVALUATION of bone fragility in endocrine disorders. Eur J Endocrinol. https://doi.org/10.1530/EJE-18-0991
Lewiecki EM (2022) Evaluating patients for secondary causes of osteoporosis. Curr Osteoporos Rep 20(1):1–12. https://doi.org/10.1007/s11914-022-00717-y
Anderson PA, Freedman BA, Brox WT, Shaffer WO (2021) Osteoporosis: recent recommendations and positions of the American Society for Bone and Mineral Research and the International Society for Clinical Densitometry. J Bone Jt Surg Am 103(8):741–747. https://doi.org/10.2106/JBJS.20.01248
Unnanuntana A, Rebolledo BJ, Khair MM, DiCarlo EF, Lane JM (2011) Diseases affecting bone quality: beyond osteoporosis. Clin Orthop Relat Res 469(8):2194–2206. https://doi.org/10.1007/s11999-010-1694-9
Seeman E (2003) Bone quality. Osteoporos Int 14(Suppl 5):S3-7. https://doi.org/10.1007/s00198-003-1465-5
Brandi ML (2009) Microarchitecture, the key to bone quality. Rheumatology (Oxford) 48(Suppl 4):3–8. https://doi.org/10.1093/rheumatology/kep273
Seeman E, Delmas PD (2006) Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med 354(21):2250–2261. https://doi.org/10.1056/NEJMra053077
Bouxsein ML (2003) Bone quality: where do we go from here? Osteoporos Int 14(Suppl 5):S118-127. https://doi.org/10.1007/s00198-003-1489-x
Hunt HB, Donnelly E (2016) Bone quality assessment techniques: geometric, compositional, and mechanical characterization from macroscale to nanoscale. Clin Rev Bone Miner Metab 14(3):133–149. https://doi.org/10.1007/s12018-016-9222-4
Seeman E (2008) Bone quality: the material and structural basis of bone strength. J Bone Miner Metab 26(1):1–8. https://doi.org/10.1007/s00774-007-0793-5
Boskey AL, Imbert L (2017) Bone quality changes associated with aging and disease: a review. Ann N Y Acad Sci 1410(1):93–106. https://doi.org/10.1111/nyas.13572
Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P (2016) Bone mechanical properties and changes with osteoporosis. Injury 47(Suppl 2):S11-20. https://doi.org/10.1016/S0020-1383(16)47003-8
Adams JE (2013) Advances in bone imaging for osteoporosis. Nat Rev Endocrinol 9(1):28–42. https://doi.org/10.1038/nrendo.2012.217
Krohn K, Schwartz EN, Chung YS, Lewiecki EM (2019) Dual-energy X-ray absorptiometry monitoring with Trabecular Bone Score: 2019 ISCD official position. J Clin Densitom 22(4):501–505. https://doi.org/10.1016/j.jocd.2019.07.006
Leslie WD, Shevroja E, Johansson H, McCloskey EV, Harvey NC, Kanis JA, Hans D (2018) Risk-equivalent T-score adjustment for using lumbar spine trabecular bone score (TBS): the Manitoba BMD registry. Osteoporos Int 29(3):751–758. https://doi.org/10.1007/s00198-018-4405-0
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: Trabecular Bone Score. J Clin Densitom 18(3):309–330. https://doi.org/10.1016/j.jocd.2015.06.008
McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Kanis JA (2015) Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 96(6):500–509. https://doi.org/10.1007/s00223-015-9980-x
Ulivieri FM, Rinaudo L (2020) Beyond bone mineral density: a new dual X-ray absorptiometry index of bone strength to predict fragility fractures, the bone strain index. Front Med 7:590139. https://doi.org/10.3389/fmed.2020.590139
Ulivieri FM, Rinaudo L, Messina C, Aliprandi A, Sconfienza LM, Sardanelli F, Cesana BM (2022) Bone Strain Index: preliminary distributional characteristics in a population of women with normal bone mass, osteopenia and osteoporosis. Radiol Med (Torino) 127(10):1151–1158. https://doi.org/10.1007/s11547-022-01543-z
Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, de Jong J, van den Bergh J (2014) High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol 10(5):304–313. https://doi.org/10.1038/nrrheum.2014.23
Engelke K, Lang T, Khosla S, Qin L, Zysset P, Leslie WD, Shepherd JA, Shousboe JT (2015) Clinical use of quantitative computed tomography-based advanced techniques in the management of osteoporosis in adults: the 2015 ISCD official positions-part III. J Clin Densitom 18(3):393–407. https://doi.org/10.1016/j.jocd.2015.06.010
Lin W, He C, Xie F, Chen T, Zheng G, Yin H, Chen H, Wang Z (2022) Discordance in lumbar bone mineral density measurements by quantitative computed tomography and dual-energy X-ray absorptiometry in postmenopausal women: a prospective comparative study. Spine J. https://doi.org/10.1016/j.spinee.2022.10.014
Radiology ACO (2018) ACR–SPR–SSR practice parameter for the performance of musculoskeletal quantitative computed tomography (QCT). American College of Radiology, Reston
Cheung WH, Hung VW, Cheuk KY, Chau WW, Tsoi KK, Wong RM, Chow SK, Lam TP, Yung PS, Law SW, Qin L (2021) Best performance parameters of HR-pQCT to predict fragility fracture: systematic review and meta-analysis. J Bone Miner Res 36(12):2381–2398. https://doi.org/10.1002/jbmr.4449
Mookiah MRK, Subburaj K, Mei K, Kopp FK, Kaesmacher J, Jungmann PM, Foehr P, Noel PB, Kirschke JS, Baum T (2018) Multidetector computed tomography imaging: effect of sparse sampling and iterative reconstruction on trabecular bone microstructure. J Comput Assist Tomogr 42(3):441–447. https://doi.org/10.1097/rct.0000000000000710
Biamonte E, Levi R, Carrone F, Vena W, Brunetti A, Battaglia M, Garoli F, Savini G, Riva M, Ortolina A, Tomei M, Angelotti G, Laino ME, Savevski V, Mollura M, Fornari M, Barbieri R, Lania AG, Grimaldi M, Politi LS, Mazziotti G (2022) Artificial intelligence-based radiomics on computed tomography of lumbar spine in subjects with fragility vertebral fractures. J Endocrinol Invest 45(10):2007–2017. https://doi.org/10.1007/s40618-022-01837-z
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577. https://doi.org/10.1148/radiol.2015151169
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue R, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762. https://doi.org/10.1038/nrclinonc.2017.141
Di Paola M, Gatti D, Viapiana O, Cianferotti L, Cavalli L, Caffarelli C, Conversano F, Quarta E, Pisani P, Girasole G, Giusti A, Manfredini M, Arioli G, Matucci-Cerinic M, Bianchi G, Nuti R, Gonnelli S, Brandi ML, Muratore M, Rossini M (2019) Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos Int 30(2):391–402. https://doi.org/10.1007/s00198-018-4686-3
Ciardo D, Pisani P, Conversano F, Casciaro S (2022) Pulse-echo measurements of bone tissues. Techniques and clinical results at the spine and femur. Adv Exp Med Biol 1364:145–162. https://doi.org/10.1007/978-3-030-91979-5_7
Diez-Perez A, Bouxsein ML, Eriksen EF, Khosla S, Nyman JS, Papapoulos S, Tang SY (2016) Technical note: recommendations for a standard procedure to assess cortical bone at the tissue-level in vivo using impact microindentation. Bone Rep 5:181–185. https://doi.org/10.1016/j.bonr.2016.07.004
Fuleihan GE-HCM, Cipriani C, Eastell R, Karonova T, Liu J-M, Minisola S, Mithal A, Moreira CA, Peacock M, Schini M, Silva B, Walker M, Zein OE, Marcocci C (2022) Classical and non-classical manifestations of primary hyperparathyroidism. J Bone Miner Res. https://doi.org/10.1002/jbmr.4679
Castellano EAR, Boriano A, Borretta V, Gennaro M, Latina A, Borretta G (2020) Radiologic manifestation of bone involvement in primary hyperparathyroidism: prevalence and clinical significance in a southern European series. End Pract 26(9):983–989. https://doi.org/10.4158/EP-2020-0095
Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV et al (1989) Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 4(3):283–291. https://doi.org/10.1002/jbmr.5650040302
Wishart JHM, Need A, Nordin BE (1990) Relationship between forearm and vertebral mineral density in postmenopausal women with primary hyperparathyroidism. Arch Intern Med 150(6):1329–1331
Parisien M, Silverberg SJ, Shane E, de la Cruz L, Lindsay R, Bilezikian JP, Dempster DW (1990) The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab 70(4):930–938. https://doi.org/10.1210/jcem-70-4-930
Vignali E, Viccica G, Diacinti D, Cetani F, Cianferotti L, Ambrogini E, Banti C, Del Fiacco R, Bilezikian JP, Pinchera A, Marcocci C (2009) Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab 94(7):2306–2312. https://doi.org/10.1210/jc.2008-2006
Khosla S, Melton LJ 3rd, Wermers RA, Crowson CS, O’Fallon W, Riggs B (1999) Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res 14(10):1700–1707. https://doi.org/10.1359/jbmr.1999.14.10.1700
Ejlsmark-Svensson H, Bislev LS, Lajlev S, Harslof T, Rolighed L, Sikjaer T, Rejnmark L (2018) Prevalence and risk of vertebral fractures in primary hyperparathyroidism: a nested case-control study. J Bone Miner Res 33(9):1657–1664. https://doi.org/10.1002/jbmr.3461
Charopoulos ITS, Trovas G, Raptou P, Kaldrymides P, Skarandavos G, Katsalira K, Lyritis GP (2006) Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocrinol Metab 91(5):1748–1753. https://doi.org/10.1210/jc.2005-2102
Stein EMSB, Boutroy S, Zhou B, Wang J, Udesky J, Zhang C, McMahon DJ, Romano M, Dworakowski E, Costa AG, Cusano N, Irani D, Cremers S, Shane E, Guo XE, Bilezikian JP (2013) Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res 28(5):1029–1040
Vu TDTWX, Wang Q, Cusano NE, Irani D, Silva BC, Ghasem-Zadeh A, Udesky J, Romano ME, Zebaze R, Jerums G, Boutroy S, Bilezikian JP, Seeman E (2013) New insights into the effects of primary hyperparathyroidism on the cortical and trabecular compartments of bone. Bone 55(1):57–63. https://doi.org/10.1016/j.bone.2013.03.009
Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, Udesky J, Cremers S, Sarquis M, Guo XD, Hans D, Bilezikian JP (2013) Trabecular bone score (TBS)–a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 98(5):1963–1970. https://doi.org/10.1210/jc.2012-4255
Jones ARSK, Harvey S, Grill V (2022) Bone mineral density compared to trabecular bone score in primary hyperparathyroidism. J Clin Med 11(2):330. https://doi.org/10.3390/jcm11020330
Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, Pepe J, Diacinti D, Piemonte S, Carnevale V, Minisola S (2013) “Trabecular Bone Score” (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone 53(1):154–159. https://doi.org/10.1016/j.bone.2012.11.041
Eller-Vainicher C, Battista C, Guarnieri V, Muscarella S, Palmieri S, Salcuni AS, Guglielmi G, Corbetta S, Minisola S, Spada A, Hendy GN, Cole DE, Chiodini I, Scillitani A (2014) Factors associated with vertebral fracture risk in patients with primary hyperparathyroidism. Eur J Endocrinol 171(3):399–406. https://doi.org/10.1530/EJE-14-0343
Tabacco G, Naciu AM, Messina C, Sanson G, Rinaudo L, Cesareo R, Falcone S, Manfrini S, Napoli N, Bilezikian JP, Ulivieri FM, Palermo A (2021) DXA-based bone strain index: a new tool to evaluate bone quality in primary hyperparathyroidism. J Clin Endocrinol Metab 106(8):2304–2312. https://doi.org/10.1210/clinem/dgab317
Anagnostis P, Vaitsi K, Veneti S, Potoupni V, Kenanidis E, Tsiridis E, Papavramidis TS, Goulis DG (2021) Efficacy of parathyroidectomy compared with active surveillance in patients with mild asymptomatic primary hyperparathyroidism: a systematic review and meta-analysis of randomized-controlled studies. J Endocrinol Invest 44(6):1127–1137. https://doi.org/10.1007/s40618-020-01447-7
Rolighed LRL, Sikjaer T, Heickendorff L, Vestergaard P, Mosekilde L, Christiansen P (2014) Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J Clin Endocrinol Metab 99(3):1072–1080. https://doi.org/10.1210/jc.2013-3978
Cipriani C, Abraham A, Silva BC, Cusano NE, Rubin MR, McMahon DJ, Zhang C, Hans D, Silverberg SJ, Bilezikian JP (2017) Skeletal changes after restoration of the euparathyroid state in patients with hypoparathyroidism and primary hyperparathyroidism. Endocrine 55(2):591–598. https://doi.org/10.1007/s12020-016-1101-8
Miguel GACF, Rodríguez JCR, Ramos MA, Pablos DL, Herrero EF, Díaz-Guerra GM (2019) Trabecular Bone Score, bone mineral density and bone markers in patients with primary hyperparathyroidism 2 years after parathyroidectomy. Horm Metab Res 51(3):186–190. https://doi.org/10.1055/a-0850-8679
Cusano NE, Rubin MR, Silva BC, Tay YD, Williams JM, Agarwal S, Omeragic B, Guo XE, Bilezikian JP (2018) Skeletal microstructure and estimated bone strength improve following parathyroidectomy in primary hyperparathyroidism. J Clin Endocrinol Metab 103(1):196–205. https://doi.org/10.1210/jc.2017-01932
Hansen S, Hauge EM, Rasmussen L, Jensen JE, Brixen K (2012) Parathyroidectomy improves bone geometry and microarchitecture in female patients with primary hyperparathyroidism: a one-year prospective controlled study using high-resolution peripheral quantitative computed tomography. J Bone Miner Res 27(5):1150–1158. https://doi.org/10.1002/jbmr.1540
Ye Z, Silverberg SJ, Sreekanta A, Tong K, Wang Y, Chang Y, Zhang M, Guyatt G, Tangamornsuksun W, Zhang Y, Manja V, Bakaa L, Couban RJ, Brandi ML, Clarke B, Khan AA, Mannstadt M, Bilezikian JP (2022) The efficacy and safety of medical and surgical therapy in patients with primary hyperparathyroidism: a systematic review and meta-analysis of randomized controlled trials. J Bone Miner Res 37(11):2351–2372. https://doi.org/10.1002/jbmr.4685
Hamdy NAT, Decallonne B, Evenepoel P, Gruson D, van Vlokhoven-Verhaegh L (2021) Burden of illness in patients with chronic hypoparathyroidism not adequately controlled with conventional therapy: a Belgium and the Netherlands survey. J Endocrinol Invest 44(7):1437–1446. https://doi.org/10.1007/s40618-020-01442-y
Bilezikian JP (2020) Hypoparathyroidism. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa113
Rubin MR, Dempster DW, Zhou H, Shane E, Nickolas T, Sliney J Jr, Silverberg SJ, Bilezikian JP (2008) Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res 23(12):2018–2024. https://doi.org/10.1359/jbmr.080803
Rubin MR, Dempster DW, Sliney J Jr, Zhou H, Nickolas TL, Stein EM, Dworakowski E, Dellabadia M, Ives R, McMahon DJ, Zhang C, Silverberg SJ, Shane E, Cremers S, Bilezikian JP (2011) PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res 26(11):2727–2736. https://doi.org/10.1002/jbmr.452
Rubin MR (2019) Skeletal manifestations of hypoparathyroidism. Bone 120:548–555. https://doi.org/10.1016/j.bone.2018.11.012
Silva BCBJ (2020) Skeletal abnormalities in hypoparathyroidism and in primary hyperparathyroidism. Rev Endocr Metab Disord. https://doi.org/10.1007/s11154-020-09614-0
Cipriani C, Pepe J, Silva BC, Rubin MR, Cusano NE, McMahon DJ, Nieddu L, Angelozzi M, Biamonte F, Diacinti D, Hans D, Minisola S, Bilezikian JP (2018) Comparative effect of rhPTH(1–84) on bone mineral density and Trabecular Bone Score in hypoparathyroidism and postmenopausal osteoporosis. J Bone Miner Res 33(12):2132–2139. https://doi.org/10.1002/jbmr.3554
Sakane ENVM, Lazaretti-Castro M, Maeda SS (2019) Predictors of poor bone microarchitecture assessed by Trabecular Bone Score in postsurgical hypoparathyroidism. J Clin Endocrinol Metab 104:5795–5803. https://doi.org/10.1210/jc.2019-00698
Saha SMV, Kandasamy D, Sreenivas V, Goswami R (2022) Vertebral fractures, trabecular bone score and their determinants in chronic hypoparathyroidism. J Endocrinol Invest. https://doi.org/10.1007/s40618-022-01818-2
Cipriani CMS, Bilezikian JP, Diacinti D, Colangelo L, Piazzolla V, Angelozzi M, Nieddu L, Pepe J, Diacinti D (2021) Vertebral fracture assessment in postmenopausal women with postsurgical hypoparathyroidism. J Clin Endocrinol Metab 106:1303–1311. https://doi.org/10.1210/clinem/dgab076
Chen QKH, Iu M-F, Nomura R, Sowa H, Yamauchi M, Tsukamoto T, Sugimoto T, Chihara K (2003) Effects of an excess and a deficiency of endogenous parathyroid hormone on volumetric bone mineral density and bone geometry determined by peripheral quantitative computed tomography in female subjects. J Clin Endocrinol Metab 88:4655–4658. https://doi.org/10.1210/jc.2003-030470
Liu JCS, Quan T, Wang Y, Wang O, Nie M, Jiang Y, Li M, Xing X, Xia W (2020) Bone microstructure of adult patients with non-surgical hypoparathyroidism assessed by high-resolution peripheral quantitative computed tomography. Osteop Int 31:2219–2230. https://doi.org/10.1007/s00198-020-05506-w
Cusano NE, Nishiyama KK, Zhang C, Rubin MR, Boutroy S, McMahon DJ, Guo XE, Bilezikian JP (2016) Noninvasive assessment of skeletal microstructure and estimated bone strength in hypoparathyroidism. J Bone Miner Res 31(2):308–316. https://doi.org/10.1002/jbmr.2609
Cusano NE, Rubin MR, Williams JM, Agarwal S, Tabacco G, Tay D, Majeed R, Omeragic B, Bilezikian JP (2020) Changes in skeletal microstructure through four continuous years of rhPTH(1–84) therapy in hypoparathyroidism. J Bone Miner Res. https://doi.org/10.1002/jbmr.4005
Agarwal S, McMahon DJ, Chen J, Brossfield A, Fernando J, Bilezikian JP, Cusano NE, Rubin MR (2023) The clinical and skeletal effects of long-term therapy of hypoparathyroidism with rhPTH(1–84). J Bone Miner Res. https://doi.org/10.1002/jbmr.4780
Williams GR, Bassett JHD (2018) Thyroid diseases and bone health. J Endocrinol Invest 41(1):99–109. https://doi.org/10.1007/s40618-017-0753-4
Mazziotti G, Frara S, Giustina A (2018) Pituitary diseases and bone. Endocr Rev 39(4):440–488. https://doi.org/10.1210/er.2018-00005
Kim SM, Ryu V, Miyashita S, Korkmaz F, Lizneva D, Gera S, Latif R, Davies TF, Iqbal J, Yuen T, Zaidi M (2021) Thyrotropin, hyperthyroidism, and bone mass. J Clin Endocrinol Metab 106(12):e4809–e4821. https://doi.org/10.1210/clinem/dgab548
Apostu D, Lucaciu O, Oltean-Dan D, Muresan AD, Moisescu-Pop C, Maxim A, Benea H (2020) the influence of thyroid pathology on osteoporosis and fracture risk: a review. Diagnostics (Basel). https://doi.org/10.3390/diagnostics10030149
Vestergaard P, Mosekilde L (2003) Hyperthyroidism, bone mineral, and fracture risk–a meta-analysis. Thyroid 13(6):585–593. https://doi.org/10.1089/105072503322238854
Ock SY, Chung YS, Choi YJ (2016) Changes in bone mineral density and trabecular bone score in Graves’ disease patients after anti-thyroid therapy. Osteoporos Sarcopenia 2(3):175–179. https://doi.org/10.1016/j.afos.2016.05.004
Nicolaisen P, Obling ML, Winther KH, Hansen S, Hermann AP, Hegedus L, Bonnema SJ, Brix TH (2021) Consequences of hyperthyroidism and its treatment for bone microarchitecture assessed by high-resolution peripheral quantitative computed tomography. Thyroid 31(2):208–216. https://doi.org/10.1089/thy.2020.0084
Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J (1993) Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. J Bone Miner Res 8(2):127–132. https://doi.org/10.1002/jbmr.5650080202
Cellini M, Rotondi M, Tanda ML, Piantanida E, Chiovato L, Beck-Peccoz P, Lania A, Mazziotti G (2021) Skeletal health in patients with differentiated thyroid carcinoma. J Endocrinol Invest 44(3):431–442. https://doi.org/10.1007/s40618-020-01359-6
Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, Wirth CD, Peeters RP, Åsvold BO, den Elzen WP, Luben RN, Imaizumi M, Bremner AP, Gogakos A, Eastell R, Kearney PM, Strotmeyer ES, Wallace ER, Hoff M, Ceresini G, Rivadeneira F, Uitterlinden AG, Stott DJ, Westendorp RG, Khaw KT, Langhammer A, Ferrucci L, Gussekloo J, Williams GR, Walsh JP, Jüni P, Aujesky D, Rodondi N, Thyroid Studies Collaboration (2015) Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA 313(20):2055–2065. https://doi.org/10.1001/jama.2015.5161
Chung CW, Choi HS, Kong SH, Park YJ, Park DJ, Ahn HY, Cho SW (2021) Measurements of bone health after thyroid-stimulating suppression therapy in postmenopausal women with differentiated thyroid carcinoma: bone mineral density versus the trabecular bone score. J Clin Med. https://doi.org/10.3390/jcm10091964
Sousa B, Silva BC, de Oliveira GT, Pires MC, Soares MMS, Kakehasi AM (2021) Trabecular bone score in women with differentiated thyroid cancer on long-term TSH-suppressive therapy. J Endocrinol Invest 44(10):2295–2305. https://doi.org/10.1007/s40618-021-01537-0
Mendonca Monteiro de Barros G, Madeira M, Vieira-Neto L, de Paula Paranhos Neto F, Carvalho Mendonca LM, Correa Barbosa Lima I, Corbo R, Fleiuss Farias ML (2016) Bone mineral density and bone microarchitecture after long-term suppressive levothyroxine treatment of differentiated thyroid carcinoma in young adult patients. J Bone Miner Metab 34(4):417–421. https://doi.org/10.1007/s00774-015-0680-4
Moon JH, Kim KM, Oh TJ, Choi SH, Lim S, Park YJ, Park DJ, Jang HC (2017) The effect of TSH suppression on vertebral trabecular bone scores in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 102(1):78–85. https://doi.org/10.1210/jc.2016-2740
De Mingo Dominguez ML, Guadalix Iglesias S, Martin-Arriscado Arroba C, Lopez Alvarez B, Martinez Diaz-Guerra G, Martinez-Pueyo JI, Ferrero Herrero E, Hawkins Carranza F (2018) Low trabecular bone score in postmenopausal women with differentiated thyroid carcinoma after long-term TSH suppressive therapy. Endocrine 62(1):166–173. https://doi.org/10.1007/s12020-018-1671-8
Mazziotti G, Formenti AM, Frara S, Olivetti R, Banfi G, Memo M, Maroldi R, Giubbini R, Giustina A (2018) High prevalence of radiological vertebral fractures in women on thyroid-stimulating hormone-suppressive therapy for thyroid carcinoma. J Clin Endocrinol Metab 103(3):956–964. https://doi.org/10.1210/jc.2017-01986
Hawkins Carranza F, Guadalix Iglesias S, De Mingo L, Dominguez M, Martin-Arriscado Arroba C, Lopez Alvarez B, Allo Miguel G, Martinez Diaz-Guerra G (2020) Trabecular bone deterioration in differentiated thyroid cancer: impact of long-term TSH suppressive therapy. Cancer Med 9(16):5746–5755. https://doi.org/10.1002/cam4.3200
Kim K, Kim IJ, Pak K, Kim SJ, Shin S, Kim BH, Kim SS, Lee BJ, Jeon YK (2018) Evaluation of bone mineral density using DXA and cQCT in postmenopausal patients under thyrotropin suppressive therapy. J Clin Endocrinol Metab 103(11):4232–4240. https://doi.org/10.1210/jc.2017-02704
Tournis S, Antoniou JD, Liakou CG, Christodoulou J, Papakitsou E, Galanos A, Makris K, Marketos H, Nikopoulou S, Tzavara I, Triantafyllopoulos IK, Dontas I, Papaioannou N, Lyritis GP, Alevizaki M (2015) Volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in women with differentiated thyroid cancer under TSH suppression. Clin Endocrinol (Oxf) 82(2):197–204. https://doi.org/10.1111/cen.12560
Vendrami C, Marques-Vidal P, Gonzalez Rodriguez E, Hans D, Waeber G, Lamy O (2022) Thyroid-stimulating hormone is associated with trabecular bone score and 5-year incident fracture risk in euthyroid postmenopausal women: the OsteoLaus cohort. Osteoporos Int 33(1):195–204. https://doi.org/10.1007/s00198-021-06081-4
Hwangbo Y, Kim JH, Kim SW, Park YJ, Park DJ, Kim SY, Shin CS, Cho NH (2016) High-normal free thyroxine levels are associated with low trabecular bone scores in euthyroid postmenopausal women. Osteoporos Int 27(2):457–462. https://doi.org/10.1007/s00198-015-3270-3
Gonzalez Rodriguez E, Stuber M, Del Giovane C, Feller M, Collet TH, Lowe AL, Blum MR, van Vliet NA, van Heemst D, Kearney PM, Gussekloo J, Mooijaart S, Westendorp RGJ, Stott DJ, Aeberli D, Bauer DC, Hans D, Rodondi N (2020) Skeletal effects of levothyroxine for subclinical hypothyroidism in older adults: a TRUST randomized trial nested study. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz058
Obling ML, Nicolaisen P, Brix TH, Winther KH, Hansen S, Hegedus L, Hermann AP, Bonnema SJ (2021) Restoration of euthyroidism in women with Hashimoto’s thyroiditis changes bone microarchitecture but not estimated bone strength. Endocrine 71(2):397–406. https://doi.org/10.1007/s12020-020-02398-y
Chiodini I, Carnevale V, Torlontano M, Fusilli S, Guglielmi G, Pileri M, Modoni S, Di Giorgio A, Liuzzi A, Minisola S, Cammisa M, Trischitta V, Scillitani A (1998) Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: study in eumenorrheic patients with Cushing’s syndrome. J Clin Endocrinol Metab 83(6):1863–1867. https://doi.org/10.1210/jcem.83.6.4880
Moraes AB, de Paula MP, de Paula Paranhos-Neto F, Cavalari EMR, de Morais FFC, Curi DSC, Lima LFC, de Mendonça LMC, Farias MLF, Madeira M, Vieira Neto L (2020) Bone evaluation by high-resolution peripheral quantitative computed tomography in patients with adrenal incidentaloma. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa263
Di Somma C, Pivonello R, Loche S, Faggiano A, Klain M, Salvatore M, Lombardi G, Colao A (2003) Effect of 2 years of cortisol normalization on the impaired bone mass and turnover in adolescent and adult patients with Cushing’s disease: a prospective study. Clin Endocrinol (Oxf) 58(3):302–308
Vinolas H, Grouthier V, Mehsen-Cetre N, Boisson A, Winzenrieth R, Schaeverbeke T, Mesguich C, Bordenave L, Tabarin A (2018) Assessment of vertebral microarchitecture in overt and mild Cushing’s syndrome using trabecular bone score. Clin Endocrinol (Oxf) 89(2):148–154. https://doi.org/10.1111/cen.13743
Ferraù F, Giovinazzo S, Messina E, Tessitore A, Vinci S, Mazziotti G, Lania A, Granata F, Cannavò S (2020) High bone marrow fat in patients with Cushing’s syndrome and vertebral fractures. Endocrine 67(1):172–179. https://doi.org/10.1007/s12020-019-02034-4
Maurice F, Dutour A, Vincentelli C, Abdesselam I, Bernard M, Dufour H, Lefur Y, Graillon T, Kober F, Cristofari P, Jouve E, Pini L, Fernandez R, Chagnaud C, Brue T, Castinetti F, Gaborit B (2018) Active Cushing syndrome patients have increased ectopic fat deposition and bone marrow fat content compared to cured patients and healthy subjects: a pilot 1H-MRS study. Eur J Endocrinol 179(5):307–317. https://doi.org/10.1530/eje-18-0318
dos Santos CV, Vieira Neto L, Madeira M, Alves Coelho MC, de Mendonca LM, Paranhos-Neto Fde P, Lima IC, Gadelha MR, Farias ML (2015) Bone density and microarchitecture in endogenous hypercortisolism. Clin Endocrinol (Oxf) 83(4):468–474. https://doi.org/10.1111/cen.12812
Apaydın T, Yavuz DG (2021) Assessment of non-traumatic vertebral fractures in Cushing’s syndrome patients. J Endocrinol Invest 44(8):1767–1773. https://doi.org/10.1007/s40618-020-01496-y
Valassi E, Santos A, Yaneva M, Toth M, Strasburger CJ, Chanson P, Wass JA, Chabre O, Pfeifer M, Feelders RA, Tsagarakis S, Trainer PJ, Franz H, Zopf K, Zacharieva S, Lamberts SW, Tabarin A, Webb SM (2011) The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol 165(3):383–392. https://doi.org/10.1530/eje-11-0272
Tauchmanova L, Pivonello R, Di Somma C, Rossi R, De Martino MC, Camera L, Klain M, Salvatore M, Lombardi G, Colao A (2006) Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab 91(5):1779–1784. https://doi.org/10.1210/jc.2005-0582
Trementino L, Appolloni G, Ceccoli L, Marcelli G, Concettoni C, Boscaro M, Arnaldi G (2014) Bone complications in patients with Cushing’s syndrome: looking for clinical, biochemical, and genetic determinants. Osteoporos Int 25(3):913–921. https://doi.org/10.1007/s00198-013-2520-5
Belaya ZE, Hans D, Rozhinskaya LY, Dragunova NV, Sasonova NI, Solodovnikov AG, Tsoriev TT, Dzeranova LK, Melnichenko GA, Dedov II (2015) The risk factors for fractures and trabecular bone-score value in patients with endogenous Cushing’s syndrome. Arch Osteoporos 10:44. https://doi.org/10.1007/s11657-015-0244-1
Eller-Vainicher C, Morelli V, Ulivieri FM, Palmieri S, Zhukouskaya VV, Cairoli E, Pino R, Naccarato A, Scillitani A, Beck-Peccoz P, Chiodini I (2012) Bone quality, as measured by trabecular bone score in patients with adrenal incidentalomas with and without subclinical hypercortisolism. J Bone Miner Res 27(10):2223–2230. https://doi.org/10.1002/jbmr.1648
Vestergaard P, Lindholm J, Jorgensen JO, Hagen C, Hoeck HC, Laurberg P, Rejnmark L, Brixen K, Kristensen LO, Feldt-Rasmussen U, Mosekilde L (2002) Increased risk of osteoporotic fractures in patients with Cushing’s syndrome. Eur J Endocrinol 146(1):51–56
Scillitani A, Mazziotti G, Di Somma C, Moretti S, Stigliano A, Pivonello R, Giustina A, Colao A (2014) Treatment of skeletal impairment in patients with endogenous hypercortisolism: when and how? Osteoporos Int 25(2):441–446. https://doi.org/10.1007/s00198-013-2588-y
Mazziotti G, Lania AG, Canalis E (2022) Skeletal disorders associated with the growth hormone–insulin- like growth factor 1 axis. Nat Rev Endocrinol 18(6):353–365. https://doi.org/10.1038/s41574-022-00649-8
Wuster C, Abs R, Bengtsson BA, Bennmarker H, Feldt-Rasmussen U, Hernberg-Stahl E, Monson JP, Westberg B, Wilton P (2001) The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J Bone Miner Res 16(2):398–405. https://doi.org/10.1359/jbmr.2001.16.2.398
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18(10):1319–1328. https://doi.org/10.1007/s00198-007-0394-0
Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, Ali AY, Abdulrafee AA, Saeda MY (2013) Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 56(2):355–362. https://doi.org/10.1016/j.bone.2013.06.029
Colao A, Di Somma C, Pivonello R, Loche S, Aimaretti G, Cerbone G, Faggiano A, Corneli G, Ghigo E, Lombardi G (1999) Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab 84(6):1919–1924. https://doi.org/10.1210/jcem.84.6.5742
Mazziotti G, Bianchi A, Bonadonna S, Nuzzo M, Cimino V, Fusco A, De Marinis L, Giustina A (2006) Increased prevalence of radiological spinal deformities in adult patients with GH deficiency: influence of GH replacement therapy. J Bone Miner Res 21(4):520–528. https://doi.org/10.1359/jbmr.060112
Bravenboer N, Holzmann P, de Boer H, Blok GJ, Lips P (1996) Histomorphometric analysis of bone mass and bone metabolism in growth hormone deficient adult men. Bone 18(6):551–557
Yang H, Yan K, Yuping X, Zhang Q, Wang L, Gong F, Zhu H, Xia W, Pan H (2019) Bone microarchitecture and volumetric bone density impairment in young male adults with childhood-onset growth hormone deficiency. Eur J Endocrinol 180(2):145–153. https://doi.org/10.1530/eje-18-0711
Gracia-Marco L, Gonzalez-Salvatierra S, Garcia-Martin A, Ubago-Guisado E, Garcia-Fontana B, Gil-Cosano JJ, Muñoz-Torres M (2021) 3D DXA hip differences in patients with acromegaly or adult growth hormone deficiency. J Clin Med. https://doi.org/10.3390/jcm10040657
Silva PPB, Amlashi FG, Yu EW, Pulaski-Liebert KJ, Gerweck AV, Fazeli PK, Lawson E, Nachtigall LB, Biller BMK, Miller KK, Klibanski A, Bouxsein M, Tritos NA (2017) Bone microarchitecture and estimated bone strength in men with active acromegaly. Eur J Endocrinol 177(5):409–420. https://doi.org/10.1530/eje-17-0468
Biller BM, Sesmilo G, Baum HB, Hayden D, Schoenfeld D, Klibanski A (2000) Withdrawal of long-term physiological growth hormone (GH) administration: differential effects on bone density and body composition in men with adult-onset GH deficiency. J Clin Endocrinol Metab 85(3):970–976. https://doi.org/10.1210/jcem.85.3.6474
Mazziotti G, Biagioli E, Maffezzoni F, Spinello M, Serra V, Maroldi R, Floriani I, Giustina A (2015) Bone turnover, bone mineral density, and fracture risk in acromegaly: a meta-analysis. J Clin Endocrinol Metab 100(2):384–394. https://doi.org/10.1210/jc.2014-2937
Madeira M, Neto LV, de Paula-Paranhos-Neto F, Barbosa-Lima IC, Carvalho-de-Mendonca LM, Gadelha MR, Fleiuss-de-Farias ML (2013) Acromegaly has a negative influence on trabecular bone, but not on cortical bone, as assessed by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 98(4):1734–1741. https://doi.org/10.1210/jc.2012-4073
Dalle Carbonare L, Micheletti V, Cosaro E, Valenti MT, Mottes M, Francia G, Davì MV (2018) Bone histomorphometry in acromegaly patients with fragility vertebral fractures. Pituitary 21(1):56–64. https://doi.org/10.1007/s11102-017-0847-1
Ueland T, Ebbesen EN, Thomsen JS, Mosekilde L, Brixen K, Flyvbjerg A, Bollerslev J (2002) Decreased trabecular bone biomechanical competence, apparent density, IGF-II and IGFBP-5 content in acromegaly. Eur J Clin Invest 32(2):122–128
Silva PPB, Pereira RMR, Takayama L, Borba CG, Duarte FH, Trarbach EB, Martin RM, Bronstein MD, Tritos NA, Jallad RS (2021) Impaired bone microarchitecture in premenopausal women with acromegaly: the possible role of Wnt signaling. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgab260
Kuzma M, Vanuga P, Sagova I, Pavai D, Jackuliak P, Killinger Z, Binkley NC, Winzenrieth R, Genant HK, Payer J (2019) Non-invasive DXA-derived bone structure assessment of acromegaly patients: a cross-sectional study. Eur J Endocrinol 180(3):201–211. https://doi.org/10.1530/eje-18-0881
Sala E, Malchiodi E, Carosi G, Verrua E, Cairoli E, Ferrante E, Filopanti M, Eller-Vainicher C, Ulivieri FM, Spada A, Arosio M, Chiodini I, Mantovani G (2021) Spine bone texture assessed by trabecular bone score in active and controlled acromegaly: a prospective study. J Endocr Soc 5(8):bvab090. https://doi.org/10.1210/jendso/bvab090
Sorohan MC, Dusceac R, Sorohan BM, Caragheorgheopol A, Poiana C (2021) Trabecular bone score and bone mineral density in acromegalic osteopathy assessment: a cross-sectional study. Arch Osteoporos 16(1):134. https://doi.org/10.1007/s11657-021-00986-7
Bioletto F, Barale M, Prencipe N, Berton AM, Parasiliti-Caprino M, Gasco V, Ghigo E, Procopio M, Grottoli S (2022) Trabecular bone score as an index of bone fragility in patients with acromegaly: a systematic review and meta-analysis. Neuroendocrinology. https://doi.org/10.1159/000528199
Maffezzoni F, Maddalo M, Frara S, Mezzone M, Zorza I, Baruffaldi F, Doglietto F, Mazziotti G, Maroldi R, Giustina A (2016) High-resolution-cone beam tomography analysis of bone microarchitecture in patients with acromegaly and radiological vertebral fractures. Endocrine 54(2):532–542. https://doi.org/10.1007/s12020-016-1078-3
Malgo F, Hamdy NA, Rabelink TJ, Kroon HM, Claessen KM, Pereira AM, Biermasz NR, Appelman-Dijkstra NM (2017) Bone material strength index as measured by impact microindentation is altered in patients with acromegaly. Eur J Endocrinol 176(3):339–347. https://doi.org/10.1530/eje-16-0808
Wassenaar MJ, Biermasz NR, Hamdy NA, Zillikens MC, van Meurs JB, Rivadeneira F, Hofman A, Uitterlinden AG, Stokkel MP, Roelfsema F, Kloppenburg M, Kroon HM, Romijn JA, Pereira AM (2011) High prevalence of vertebral fractures despite normal bone mineral density in patients with long-term controlled acromegaly. Eur J Endocrinol 164(4):475–483. https://doi.org/10.1530/eje-10-1005
Calatayud M, Pérez-Olivares Martín L, Librizzi MS, Lora Pablos D, González Méndez V, Aramendi Ramos M, Martínez Diaz-Guerra G, Hawkins F (2021) Trabecular bone score and bone mineral density in patients with long-term controlled acromegaly. Clin Endocrinol (Oxf). https://doi.org/10.1111/cen.14439
Godang K, Lekva T, Normann KR, Olarescu NC, Oystese KAB, Kolnes A, Ueland T, Bollerslev J, Heck A (2019) Hip structure analyses in acromegaly: decrease of cortical bone thickness after treatment: a longitudinal cohort study. JBMR Plus 3(12):e10240. https://doi.org/10.1002/jbm4.10240
Chiloiro S, Giampietro A, Frara S, Bima C, Donfrancesco F, Fleseriu CM, Pontecorvi A, Giustina A, Fleseriu M, De Marinis L, Bianchi A (2020) Effects of pegvisomant and pasireotide LAR on vertebral fractures in acromegaly resistant to first-generation SRLs. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz054
Mazziotti G, Battista C, Maffezzoni F, Chiloiro S, Ferrante E, Prencipe N, Grasso L, Gatto F, Olivetti R, Arosio M, Barale M, Bianchi A, Cellini M, Chiodini I, De Marinis L, Del Sindaco G, Di Somma C, Ferlin A, Ghigo E, Giampietro A, Grottoli S, Lavezzi E, Mantovani G, Morenghi E, Pivonello R, Porcelli T, Procopio M, Pugliese F, Scillitani A, Lania AG (2020) Treatment of acromegalic osteopathy in real-life clinical practice: the BAAC (Bone Active Drugs in Acromegaly) study. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa363
Vitali E, Palagano E, Schiavone ML, Mantovani G, Sobacchi C, Mazziotti G, Lania A (2022) Direct effects of octreotide on osteoblast cell proliferation and function. J Endocrinol Invest 45(5):1045–1057. https://doi.org/10.1007/s40618-022-01740-7
Pelsma ICM, Biermasz NR, Pereira AM, van Furth WR, Appelman-Dijkstra NM, Kloppenburg M, Kroon HM, Claessen K (2020) Progression of vertebral fractures in long-term controlled acromegaly: a 9-year follow-up study. Eur J Endocrinol 183(4):427–437. https://doi.org/10.1530/eje-20-0415
Rolla M, Halupczok-Żyła J, Jawiarczyk-Przybyłowska A, Bolanowski M (2020) Bone densitometry by radiofrequency echographic multi-spectrometry (REMS) in acromegaly patients. Endokrynol Pol 71(6):524–531. https://doi.org/10.5603/EP.a2020.0056
Rochira V, Antonio L, Vanderschueren D (2018) EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology 6(2):272–285. https://doi.org/10.1111/andr.12470
Corona G, Vena W, Pizzocaro A, Giagulli VA, Francomano D, Rastrelli G, Mazziotti G, Aversa A, Isidori AM, Pivonello R, Vignozzi L, Mannucci E, Maggi M, Ferlin A (2022) Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest 45(5):911–926. https://doi.org/10.1007/s40618-021-01702-5
Ferlin A, Selice R, Carraro U, Foresta C (2013) Testicular function and bone metabolism–beyond testosterone. Nat Rev Endocrinol 9(9):548–554. https://doi.org/10.1038/nrendo.2013.135
Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC (2017) Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 97(1):135–187. https://doi.org/10.1152/physrev.00033.2015
Rochira V, Kara E, Carani C (2015) The endocrine role of estrogens on human male skeleton. Int J Endocrinol 2015:165215. https://doi.org/10.1155/2015/165215
Porcelli T, Maffezzoni F, Pezzaioli LC, Delbarba A, Cappelli C, Ferlin A (2020) Management of endocrine disease: male osteoporosis: diagnosis and management - should the treatment and the target be the same as for female osteoporosis? Eur J Endocrinol 183(3):R75-r93. https://doi.org/10.1530/eje-20-0034
Vanderschueren D, Pye SR, Venken K, Borghs H, Gaytant J, Huhtaniemi IT, Adams JE, Ward KA, Bartfai G, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Silman AJ, Wu FC, O’Neill TW, Boonen S (2010) Gonadal sex steroid status and bone health in middle-aged and elderly European men. Osteoporos Int 21(8):1331–1339. https://doi.org/10.1007/s00198-009-1144-2
Greendale GA, Edelstein S, Barrett-Connor E (1997) Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12(11):1833–1843. https://doi.org/10.1359/jbmr.1997.12.11.1833
Bjørnerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Jørgensen L, Øian P, Seeman E, Straume B (2007) A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men: the Tromso Study. Eur J Endocrinol 157(1):119–125. https://doi.org/10.1530/eje-07-0032
Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW, Hirsch SC, Linker A, Perros N, Servais AB, Taylor AP, Webb ML, Youngner JM, Yu EW (2016) Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest 126(3):1114–1125. https://doi.org/10.1172/jci84137
Isidori AM, Aversa A, Calogero A, Ferlin A, Francavilla S, Lanfranco F, Pivonello R, Rochira V, Corona G, Maggi M (2022) Adult- and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J Endocrinol Invest. https://doi.org/10.1007/s40618-022-01859-7
Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA (2018) Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 103(5):1715–1744. https://doi.org/10.1210/jc.2018-00229
Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, Cocci A, Corona G, Dimitropoulos K, Gül M, Hatzichristodoulou G, Jones TH, Kadioglu A, Martínez Salamanca JI, Milenkovic U, Modgil V, Russo GI, Serefoglu EC, Tharakan T, Verze P, Minhas S (2021) European Association of Urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol 80(3):333–357. https://doi.org/10.1016/j.eururo.2021.06.007
Corona G, Filippi S, Bianchi N, Dicuio M, Rastrelli G, Concetti S, Sforza A, Maggi M (2021) Cardiovascular risks of androgen deprivation therapy for prostate cancer. World J Mens Health 39(3):429–443. https://doi.org/10.5534/wjmh.200109
Vena W, Pizzocaro A, Indirli R, Amer M, Maffezzoni F, Delbarba A, Leonardi L, Balzarini L, Ulivieri FM, Ferlin A, Mantovani G, Lania AG, Ferrante E, Mazziotti G (2020) Prevalence and determinants of radiological vertebral fractures in patients with Klinefelter syndrome. Andrology 8(6):1699–1704. https://doi.org/10.1111/andr.12841
Mazziotti G, Vena W, Pedersini R, Piccini S, Morenghi E, Cosentini D, Zucali P, Torrisi R, Sporeni S, Simoncini EL, Maroldi R, Balzarini L, Lania AG, Berruti A (2022) Prediction of vertebral fractures in cancer patients undergoing hormone deprivation therapies: reliability of who fracture risk assessment tool (frax) and bone mineral density in real-life clinical practice. J Bone Oncol 33:1021. https://doi.org/10.1016/j.jbo.2022.100421
Benito M, Gomberg B, Wehrli FW, Weening RH, Zemel B, Wright AC, Song HK, Cucchiara A, Snyder PJ (2003) Deterioration of trabecular architecture in hypogonadal men. J Clin Endocrinol Metab 88(4):1497–1502. https://doi.org/10.1210/jc.2002-021429
Zhang XH, Liu XS, Vasilic B, Wehrli FW, Benito M, Rajapakse CS, Snyder PJ, Guo XE (2008) In vivo microMRI-based finite element and morphological analyses of tibial trabecular bone in eugonadal and hypogonadal men before and after testosterone treatment. J Bone Miner Res 23(9):1426–1434. https://doi.org/10.1359/jbmr.080405
Shanbhogue VV, Hansen S, Jørgensen NR, Brixen K, Gravholt CH (2014) Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in Klinefelter syndrome. J Bone Miner Res 29(11):2474–2482. https://doi.org/10.1002/jbmr.2272
Piot A, Plotton I, Boutroy S, Bacchetta J, Ailloud S, Lejeune H, Chapurlat RD, Szulc P, Confavreux CB (2022) Klinefelter bone microarchitecture evolution with testosterone replacement therapy. Calcif Tissue Int 111(1):35–46. https://doi.org/10.1007/s00223-022-00956-2
Varimo T, Miettinen PJ, Laine T, Salonen P, Tenhola S, Voutilainen R, Huopio H, Hero M, Raivio T (2021) Bone structure assessed with pQCT in prepubertal males with delayed puberty or congenital hypogonadotropic hypogonadism. Clin Endocrinol (Oxf) 95(1):107–116. https://doi.org/10.1111/cen.14466
Tahani N, Nieddu L, Prossomariti G, Spaziani M, Granato S, Carlomagno F, Anzuini A, Lenzi A, Radicioni AF, Romagnoli E (2018) Long-term effect of testosterone replacement therapy on bone in hypogonadal men with Klinefelter Syndrome. Endocrine 61(2):327–335. https://doi.org/10.1007/s12020-018-1604-6
Vena W, Carrone F, Delbarba A, Akpojiyovbi O, Pezzaioli LC, Facondo P, Cappelli C, Leonardi L, Balzarini L, Farina D, Pizzocaro A, Lania AG, Mazziotti G, Ferlin A (2023) Body composition, trabecular bone score and vertebral fractures in subjects with Klinefelter syndrome. J Endocrinol Invest 46(2):297–304. https://doi.org/10.1007/s40618-022-01901-8
Ostertag A, Papadakis GE, Collet C, Trabado S, Maione L, Pitteloud N, Bouligand J, De Vernejoul MC, Cohen-Solal M, Young J (2021) Compromised volumetric bone density and microarchitecture in men with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab 106(9):e3312–e3326. https://doi.org/10.1210/clinem/dgab169
Blüher M (2009) Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 117(6):241–250. https://doi.org/10.1055/s-0029-1192044
Pizzocaro A, Vena W, Condorelli R, Radicioni A, Rastrelli G, Pasquali D, Selice R, Ferlin A, Foresta C, Jannini EA, Maggi M, Lenzi A, Pivonello R, Isidori AM, Garolla A, Francavilla S, Corona G (2020) Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest 43(12):1675–1687. https://doi.org/10.1007/s40618-020-01299-1
Zitzmann M, Aksglaede L, Corona G, Isidori AM, Juul A, T’Sjoen G, Kliesch S, D’Hauwers K, Toppari J, Słowikowska-Hilczer J, Tüttelmann F, Ferlin A (2021) European academy of andrology guidelines on Klinefelter Syndrome Endorsing Organization: European Society of Endocrinology. Andrology 9(1):145–167. https://doi.org/10.1111/andr.12909
Corona G, Rastrelli G, Vignozzi L, Barbonetti A, Sforza A, Mannucci E, Maggi M (2021) The Role of testosterone treatment in patients with metabolic disorders. Expert Rev Clin Pharmacol 14(9):1091–1103. https://doi.org/10.1080/17512433.2021.1938548
Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, Lewis CE, Barrett-Connor E, Schwartz AV, Lee DC, Bhasin S, Cunningham GR, Gill TM, Matsumoto AM, Swerdloff RS, Basaria S, Diem SJ, Wang C, Hou X, Cifelli D, Dougar D, Zeldow B, Bauer DC, Keaveny TM (2017) Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med 177(4):471–479. https://doi.org/10.1001/jamainternmed.2016.9539
Cauley JA, Ellenberg SS, Schwartz AV, Ensrud KE, Keaveny TM, Snyder PJ (2021) Effect of testosterone treatment on the trabecular bone score in older men with low serum testosterone. Osteoporos Int 32(11):2371–2375. https://doi.org/10.1007/s00198-021-06022-1
Colleluori G, Aguirre L, Napoli N, Qualls C, Villareal DT, Armamento-Villareal R (2021) Testosterone therapy effects on bone mass and turnover in hypogonadal men with type 2 diabetes. J Clin Endocrinol Metab 106(8):e3058–e3068. https://doi.org/10.1210/clinem/dgab181
Leanza G, Maddaloni E, Pitocco D, Conte C, Palermo A, Maurizi AR, Pantano AL, Suraci C, Altomare M, Strollo R, Manfrini S, Pozzilli P, Schwartz AV, Napoli N (2019) Risk factors for fragility fractures in type 1 diabetes. Bone 125:194–199. https://doi.org/10.1016/j.bone.2019.04.017
Napoli N, Schwartz AV, Palermo L, Jin JJ, Wustrack R, Cauley JA, Ensrud KE, Kelly M, Black DM (2013) Risk factors for subtrochanteric and diaphyseal fractures: the study of osteoporotic fractures. J Clin Endocrinol Metab 98(2):659–667. https://doi.org/10.1210/jc.2012-1896
Napoli N, Strotmeyer ES, Ensrud KE, Sellmeyer DE, Bauer DC, Hoffman AR, Dam TT, Barrett-Connor E, Palermo L, Orwoll ES, Cummings SR, Black DM, Schwartz AV (2014) Fracture risk in diabetic elderly men: the MrOS study. Diabetologia 57(10):2057–2065. https://doi.org/10.1007/s00125-014-3289-6
Napoli N, Conte C, Pedone C, Strotmeyer ES, Barbour KE, Black DM, Samelson EJ, Schwartz AV (2019) Effect of insulin resistance on BMD and fracture risk in older adults. J Clin Endocrinol Metab 104(8):3303–3310. https://doi.org/10.1210/jc.2018-02539
Hofbauer LC, Busse B, Eastell R, Ferrari S, Frost M, Müller R, Burden AM, Rivadeneira F, Napoli N, Rauner M (2022) Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol 10(3):207–220. https://doi.org/10.1016/s2213-8587(21)00347-8
Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM (2014) Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab 25(1):15–22. https://doi.org/10.1016/j.tem.2013.08.002
Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K (2016) Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol 174(2):115–124. https://doi.org/10.1530/eje-15-0860
Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, Forti G, Mannucci E, Maggi M (2011) Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl 34(6 Pt 1):528–540. https://doi.org/10.1111/j.1365-2605.2010.01117.x
Napoli N, Strollo R, Defeudis G, Leto G, Moretti C, Zampetti S, D’Onofrio L, Campagna G, Palermo A, Greto V, Manfrini S, Hawa MI, Leslie RD, Pozzilli P, Buzzetti R (2018) Serum sclerostin and bone turnover in latent autoimmune diabetes in adults. J Clin Endocrinol Metab 103(5):1921–1928. https://doi.org/10.1210/jc.2017-02274
Piccoli A, Cannata F, Strollo R, Pedone C, Leanza G, Russo F, Greto V, Isgrò C, Quattrocchi CC, Massaroni C, Silvestri S, Vadalà G, Bisogno T, Denaro V, Pozzilli P, Tang SY, Silva MJ, Conte C, Papalia R, Maccarrone M, Napoli N (2020) Sclerostin regulation, microarchitecture, and advanced glycation end-products in the bone of elderly women with type 2 diabetes. J Bone Miner Res 35(12):2415–2422. https://doi.org/10.1002/jbmr.4153
Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL (2017) Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol 176(3):R137-r157. https://doi.org/10.1530/eje-16-0652
Epstein S, Defeudis G, Manfrini S, Napoli N, Pozzilli P (2016) Diabetes and disordered bone metabolism (diabetic osteodystrophy): time for recognition. Osteoporos Int 27(6):1931–1951. https://doi.org/10.1007/s00198-015-3454-x
Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML (2015) Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int 26(2):673–679. https://doi.org/10.1007/s00198-014-2927-7
Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM (2013) Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res 28(2):313–324. https://doi.org/10.1002/jbmr.1763
Karim L, Moulton J, Van Vliet M, Velie K, Robbins A, Malekipour F, Abdeen A, Ayres D, Bouxsein ML (2018) Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone 114:32–39. https://doi.org/10.1016/j.bone.2018.05.030
Hunt HB, Torres AM, Palomino PM, Marty E, Saiyed R, Cohn M, Jo J, Warner S, Sroga GE, King KB, Lane JM, Vashishth D, Hernandez CJ, Donnelly E (2019) Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus. J Bone Miner Res 34(7):1191–1206. https://doi.org/10.1002/jbmr.3711
Napoli N, Schwartz AV, Schafer AL, Vittinghoff E, Cawthon PM, Parimi N, Orwoll E, Strotmeyer ES, Hoffman AR, Barrett-Connor E, Black DM (2018) Vertebral fracture risk in diabetic elderly men: the MrOS study. J Bone Miner Res 33(1):63–69. https://doi.org/10.1002/jbmr.3287
Leslie WD, Aubry-Rozier B, Lamy O, Hans D (2013) TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 98(2):602–609. https://doi.org/10.1210/jc.2012-3118
Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, Shin CS (2015) Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab 100(2):475–482. https://doi.org/10.1210/jc.2014-2047
Caffarelli C, TomaiPitinca MD, Al Refaie A, Ceccarelli E, Gonnelli S (2022) Ability of radiofrequency echographic multispectrometry to identify osteoporosis status in elderly women with type 2 diabetes. Aging Clin Exp Res 34(1):121–127. https://doi.org/10.1007/s40520-021-01889-w
Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, Eastell R, El-Hajj Fuleihan G, Josse R, Kendler DL, Kraenzlin M, Suzuki A, Pierroz DD, Schwartz AV, Leslie WD (2018) Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 29(12):2585–2596. https://doi.org/10.1007/s00198-018-4650-2
Eastell R, Vittinghoff E, Lui LY, Ewing SK, Schwartz AV, Bauer DC, Black DM, Bouxsein ML (2022) Diabetes mellitus and the benefit of antiresorptive therapy on fracture risk. J Bone Miner Res. https://doi.org/10.1002/jbmr.4697
Schwartz AV, Pavo I, Alam J, Disch DP, Schuster D, Harris JM, Krege JH (2016) Teriparatide in patients with osteoporosis and type 2 diabetes. Bone 91:152–158. https://doi.org/10.1016/j.bone.2016.06.017
Funding
The authors declare that they have no financial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All the authors declare that they have no conflicts of interest.
Informed consent
For this type of study, formal consent is not required.
Research involving human participants and/or animals
The authors received the permission by two patients followed-up in the outpatient bone clinic for using anonymously for research purposes the images of their DXA and CT exams.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cianferotti, L., Cipriani, C., Corbetta, S. et al. Bone quality in endocrine diseases: determinants and clinical relevance. J Endocrinol Invest 46, 1283–1304 (2023). https://doi.org/10.1007/s40618-023-02056-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02056-w