Abstract
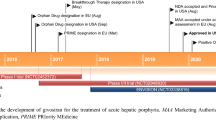
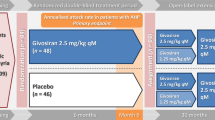
In November 2019 givosiran became the second small interfering RNA (siRNA)-based drug to receive US Food and Drug Administration (FDA) approval, it has been developed for the treatment of acute intermittent porphyria (AIP), a disorder characterized by life-threatening acute neurovisceral attacks. The porphyrias are a group of disorders in which enzymatic deficiencies in heme production lead to toxic accumulation of delta-aminolevulinic acid (ALA) and porphobilinogen (PBG), which are involved in the neurovisceral attacks. Givosiran acts as a conventional siRNA to trigger RNA interference (RNAi)-mediated gene silencing on delta-ALA synthase 1 (ALAS1), thus returning ALA and PBG metabolites to the physiological level to attenuate further neurotoxicity. Givosiran makes use of a new hepatic-delivery system that conjugates three GalNac (N-acetylgalactosamine) molecules to the siRNA passenger strand. GalNac binds to the liver asialoglycoprotein receptor, favoring the internalization of these GalNac-conjugated siRNAs into the hepatic cells. In a phase I study, subcutaneous monthly administration of givosiran 2.5 mg/kg reduced > 90% of ALA and PBG content. This siRNA is being analyzed in ENVISION (NCT03338816), a phase III, multicenter, placebo-controlled randomized controlled trial. In preliminary results, givosiran achieved clinical endpoints for AIP, reducing urinary ALA levels, and presented a safety profile that enabled further drug development. The clinical performance of givosiran revealed that suppression of ALAS1 by GalNac-decorated siRNAs represents an additional approach for the treatment of patients with AIP that manifests recurrent acute neurovisceral attacks.


Similar content being viewed by others
References
Sardh E, Harper P, Balwani M, Stein P, Rees D, Bissell DM, et al. Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N Engl J Med. 2019;380:549–58. https://doi.org/10.1056/NEJMoa1807838.
Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med [Internet]. 2017;377:2101. https://www.ncbi.nlm.nih.gov/pubmed/29166231. Accessed 23 Nov 2017
Balwani M, Desnick RJ. The porphyrias: advances in diagnosis and treatment. Hematol. Am Soc Hematol Educ Progr. 2012;2012:19–27. https://www.ncbi.nlm.nih.gov/pubmed/23233556. Accessed 13 Dec 2017
Nick L. Born to the purple: the story of porphyria—Scientific American. Sci Am. 2012. https://www.scientificamerican.com/article/born-to-the-purple-the-st/. Accessed 16 Dec 2002
Dayan FE, Dayan EA. Porphyrins: one ring in the colors of life. Am Sci. 2011;99:236–44.
Bren KL, Eisenberg R, Gray HB. Discovery of the magnetic behavior of hemoglobin: a beginning of bioinorganic chemistry. Proc Natl Acad Sci. 2015;112:13123–7.
Ramanujam VM, Anderson KE. Porphyria diagnostics-Part 1: a brief overview of the porphyrias. Curr Protoc Hum Genet. 2015;86:17.20.1–26. https://doi.org/10.1002/0471142905.hg1720s86.
Wang B, Rudnick S, Cengia B, Bonkovsky HL. Acute hepatic porphyrias: review and recent progress. Hepatol Commun [Internet]. 2019;3:193–206. https://www.ncbi.nlm.nih.gov/pubmed/30766957. Accessed 16 Feb 2019
Pischik E, Kauppinen R. Neurological manifestations of acute intermittent porphyria. Cell Mol Biol. (Noisy-le-grand). 2009;55:72–83.
Singal AK, Parker C, Bowden C, Thapar M, Liu L, McGuire BM. Liver transplantation in the management of porphyria. Hepatology [Internet]. 2014;60:1082–9. https://www.ncbi.nlm.nih.gov/pubmed/24700519. Accessed 05 Apr 2014
Maranda EL, Heifetz R, Estes WA, Cortizo J, Shareef S, Jimenez JJ. Porphyria and vampirism—a myth, sensationalized. JAMA Dermatol. 2016;152:975.
Bissell DM, Wang B. Acute Hepatic Porphyria. J Clin Transl Hepatol [Internet]. 2015;3:17–26. https://www.ncbi.nlm.nih.gov/pubmed/26357631. Accessed 12 Sept 2015
Elder G, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis [Internet]. 2013;36:849–57. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23114748. Accessed 02 Nov 2012
Floderus Y, Shoolingin-Jordan PM, Harper P. Acute intermittent porphyria in Sweden. Molecular, functional and clinical consequences of some new mutations found in the porphobilinogen deaminase gene. Clin Genet [Internet]. 2002;62:288–97. https://www.ncbi.nlm.nih.gov/pubmed/12372055. Accessed 10 Oct 2002
Besur S, Hou W, Schmeltzer P, Bonkovsky H. Clinically important features of porphyrin and heme metabolism and the porphyrias. Metabolites. 2014;4:977–1006.
Lenglet H, Schmitt C, Grange T, Manceau H, Karboul N, Bouchet-Crivat F, et al. From a dominant to an oligogenic model of inheritance with environmental modifiers in acute intermittent porphyria. Hum Mol Genet [Internet]. 2018;27:1164–73. https://www.ncbi.nlm.nih.gov/pubmed/29360981. Accessed 24 Jan 2018
Yasuda M, Chen B, Desnick RJ. Recent advances on porphyria genetics: inheritance, penetrance and molecular heterogeneity, including new modifying/causative genes. Mol Genet Metab [Internet]. 2018. https://www.ncbi.nlm.nih.gov/pubmed/30594473. Accessed 31 Dec 2018
Manceau H, Gouya L, Puy H. Acute hepatic and erythropoietic porphyrias. Curr Opin Hematol. 2017;24:198–207.
Stein PE, Badminton MN, Rees DC. Update review of the acute porphyrias. Br J Haematol. 2017;176:527–38.
Mustajoki P, Nordmann Y. Early administration of heme arginate for acute porphyric attacks. Arch Intern Med [Internet]. 1993;153:2004–8. https://www.ncbi.nlm.nih.gov/pubmed/8357285. Accessed 13 Sept 1993
Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet [Internet]. 2015;8:201–14. https://www.ncbi.nlm.nih.gov/pubmed/26366103. Accessed 15 Sept 2015
Nikam RR, Gore KR. Journey of siRNA: clinical developments and targeted delivery. Nucleic Acid Ther. 2018;28:209–24.
Yasuda M, Gan L, Chen B, Kadirvel S, Yu C, Phillips JD, et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc Natl Acad Sci USA [Internet]. 2014;111:7777–82. https://www.ncbi.nlm.nih.gov/pubmed/24821812. Accessed 14 May 2014
Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res [Internet]. 2018;46:1584–600. https://www.ncbi.nlm.nih.gov/pubmed/29240946. Accessed 15 Dec 2017
Chan A, Liebow A, Yasuda M, Gan L, Racie T, Maier M, et al. Preclinical development of a subcutaneous ALAS1 RNAi therapeutic for treatment of hepatic porphyrias using circulating RNA quantification. Mol Ther Nucleic Acids. 2015;4:e263.
Huang Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol Ther Nucleic Acids. 2017;6:116–32. https://doi.org/10.1016/j.omtn.2016.12.003.
Matsuda S, Keiser K, Nair JK, Charisse K, Manoharan RM, Kretschmer P, et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem Biol. 2015;10:1181–7.
Khorev O, Stokmaier D, Schwardt O, Cutting B, Ernst B. Trivalent, Gal/GalNAc-containing ligands designed for the asialoglycoprotein receptor. Bioorg Med Chem. 2008;16:5216–31.
Pricer WE, Hudgin RL, Ashwell G, Stockert RJ, Morell AG. [87] A membrane receptor protein for asialoglycoproteins. Methods Enzymol. 1974;34:688–91.
Rensen PCN, Van Leeuwen SH, Sliedregt LAJM, Van Berkel TJC, Biessen EAL. Design and synthesis of novel N-acetylgalactosamine-terminated glycolipids for targeting of lipoproteins to the hepatic asialoglycoprotein receptor. J Med Chem. 2004;47:5798–808.
Titze-de-Almeida R, David C, Titze-de-Almeida SS. The race of ten synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res [Internet]. 2017;34:1339–63. https://www.ncbi.nlm.nih.gov/pubmed/28389707. Accessed 09 Apr 2017
Cummins LL, Owens SR, Risen LM, Lesnik EA, Freier SM, Mc Gee D, et al. Characterization of fully 2′-modified oligoribonucleotide hetero-and homoduplex hybridization andnuclease sensitivity. Nucleic Acids Res. 1995;23:2019–24.
Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–71.
Takahashi M, Minakawa N, Matsuda A. Synthesis and characterization of 2′-modified-4′-thioRNA: a comprehensive comparison of nuclease stability. Nucleic Acids Res. 2009;37:1353–62.
Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, et al. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem. 2005;48(4):901–4. https://doi.org/10.1021/jm049167j.
Prakash TP, Kinberger GA, Murray HM, Chappell A, Riney S, Graham MJ, et al. Synergistic effect of phosphorothioate, 5′-vinylphosphonate and GalNAc modifications for enhancing activity of synthetic siRNA. Lett: Bioorg Med Chem; 2016.
Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61. https://doi.org/10.1021/ja505986a.
Nair JK, Attarwala H, Sehgal A, Wang Q, Aluri K, Zhang X, et al. Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc-siRNA conjugates. Nucleic Acids Res. 2017;45:10969–77.
Foster DJ, Brown CR, Shaikh S, Trapp C, Schlegel MK, Qian K, et al. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol Ther. 2018;26:708–17.
Balwani M, Sardh E, Gouya L, Rees DC, Stein P, Stölzel U et al. Disease characteristics of patients with acute hepatic porphyria patients: ENVISION, a Phase 3 global, multicenter, randomized, double-blind, placebo-controlled trial. https://www.alnylam.com/wp-content/uploads/2019/09/ICPP_Balwani_ENVISION-Disease-Characteristics.pdf. Accessed 10 Oct 2019
Alnylam_Pharmaceuticals. ENVISION, a Phase 3 study to evaluate the efficacy and safety of givosiran, an investigational RNAi therapeutic targeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. ICPP|Milan, Italy [Internet]. 2019. https://www.alnylam.com/wp-content/uploads/2019/09/ICPP_Gouya_ENVISION.pdf. Accessed 10 Sept 2019
Abstracts of The International Liver CongressTM 2019—54th annual meeting of the European Association for the Study of the Liver April 10–14 Vienna, Austria. J Hepatol. 2019;70:e1–e952. 2019. No Title [Internet]. https://www.journal-of-hepatology.eu/article/S0168-8278(19)30196-5/pdf. Accessed 10 Jul 2019
Balwani M, Gouya L, Rees DC, Stein P, Stölzel U, Aguilera Peiro P, et al. ENVISION, a Phase 3 study to evaluate the efficacy andsafety of givosiran, an investigational RNAi therapeutictargeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. 2019. https://www.alnylam.com/wp-content/uploads/2019/04/Balwani_ENVISION_EASL_FINAL2-2.pdf. Accessed 08 Oct 2019.
Titze-de-Almeida SS, Brandão PRP, Faber I, Titze-de-Almeida R. Leading RNA interference therapeutics part 1: silencing hereditary transthyretin amyloidosis, with a focus on patisiran. Mol Diagn Ther. 2019. https://doi.org/10.1007/s40291-019-00434-w(Epub ahead of print).
Acknowledgements
Pedro Renato de Paula Brandão, Simoneide S. Titze-de-Almeida, and Ricardo Titze-de-Almeida are members of the Network for Translational Neuroscience-International Consortium for Academic Cooperation in Experimental and Clinical Studies Regarding Neurodegenerative Diseases (http://dgp.cnpq.br/dgp/espelhogrupo/5933421119277338).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of interest
Pedro Renato de Paula Brandão, Simoneide S. Titze-de-Almeida, and Ricardo Titze-de-Almeida have no conflicts of interest that are directly relevant to the content of this study.
Rights and permissions
About this article
Cite this article
de Paula Brandão, P.R., Titze-de-Almeida, S.S. & Titze-de-Almeida, R. Leading RNA Interference Therapeutics Part 2: Silencing Delta-Aminolevulinic Acid Synthase 1, with a Focus on Givosiran. Mol Diagn Ther 24, 61–68 (2020). https://doi.org/10.1007/s40291-019-00438-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-019-00438-6