Abstract
Background
While the efficacy and safety of zanubrutinib have been established in relapsed or refractory chronic lymphocytic leukemia, the evidence on cost effectiveness is still lacking.
Objective
We aimed to evaluate the cost effectiveness of zanubrutinib versus ibrutinib in relapsed or refractory chronic lymphocytic leukemia from the commercial payer perspective in the USA.
Methods
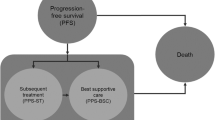
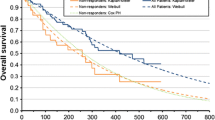
A partitioned survival model was developed based on survival curves from the phase III ALPINE trial. We reconstructed patient-level data for each curve and conducted a parametric estimation to incorporate long-term clinical outcomes and treatment costs into the model. Medical costs and utilities were obtained from public data and previous cost-effectiveness studies. A discount rate of 3.0% per annum was applied and costs were adjusted to 2023 US dollars. The incremental cost-effectiveness ratio was calculated by dividing the incremental costs of zanubrutinib over ibrutinib by the incremental life-years or quality-adjusted life-years. Deterministic and probabilistic sensitivity analyses were performed to examine the robustness of the results.
Results
Over a 10-year analysis period, the incremental cost-effectiveness ratio of zanubrutinib versus ibrutinib was $91,260 per life-year gained and $120,634 per quality-adjusted life-year gained, making it cost effective within a threshold of $150,000 per quality-adjusted life-year gained. The incremental cost-effectiveness ratio was most sensitive to drug acquisition costs and progression-free survival distributions, and the probability of zanubrutinib being cost effective was approximately 52.8%, with a 30.0% likelihood of dominance.
Conclusions
Zanubrutinib is likely to be cost effective versus ibrutinib in relapsed or refractory chronic lymphocytic leukemia in the USA, but the high threshold should be noted. Our findings may provide a basis for pricing strategy and reimbursement decisions for zanubrutinib.




Similar content being viewed by others
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. https://doi.org/10.3322/caac.21763.
Moreno C. Standard treatment approaches for relapsed/refractory chronic lymphocytic leukemia after frontline chemoimmunotherapy. Hematol Am Soc Hematol Educ Program. 2020;2020(1):33–40. https://doi.org/10.1182/hematology.2020000086.
Woyach JA. Management of relapsed/refractory chronic lymphocytic leukemia. Am J Hematol. 2022;97(Suppl. 2):S11–8. https://doi.org/10.1002/ajh.26683.
Raedler LA. Imbruvica (ibrutinib), first-in-class Bruton’s tyrosine kinase inhibitor, receives expanded indications for patients with relapsed chronic lymphocytic leukemia. Am Health Drug Benefits. 2015;8(Spec Future):66–9.
Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63. https://doi.org/10.1002/ajh.25638.
Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–9. https://doi.org/10.3324/haematol.2017.182907.
Guo Y, Liu Y, Hu N, Yu D, Zhou C, Shi G, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem. 2019;62(17):7923–40. https://doi.org/10.1021/acs.jmedchem.9b00687.
Brown JR, Eichhorst B, Hillmen P, Jurczak W, Kaźmierczak M, Lamanna N, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319–32. https://doi.org/10.1056/NEJMoa2211582.
Wierda WG, Brown J, Abramson JS, Awan F, Bilgrami SF, Bociek G, et al. NCCN Guidelines® insights: chronic lymphocytic leukemia/small lymphocytic lymphoma, Version 3.2022. J Natl Compr Canc Netw. 2022;20(6):622–34. https://doi.org/10.6004/jnccn.2022.0031.
Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33. https://doi.org/10.1016/j.annonc.2020.09.019.
Chen Q, Jain N, Ayer T, Wierda WG, Flowers CR, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017;35(2):166–74. https://doi.org/10.1200/JCO.2016.68.2856.
Mato AR, Thompson M, Allan JN, Brander DM, Pagel JM, Ujjani CS, et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica. 2018;103(9):1511–7. https://doi.org/10.3324/haematol.2018.193615.
Winqvist M, Asklid A, Andersson PO, Karlsson K, Karlsson C, Lauri B, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program: a study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 2016;101(12):1573–80. https://doi.org/10.3324/haematol.2016.144576.
Abrisqueta P, Loscertales J, Terol MJ, Ramírez Payer Á, Ortiz M, Pérez I, et al. Real-world characteristics and outcome of patients treated with single-agent ibrutinib for chronic lymphocytic leukemia in Spain (IBRORS-LLC Study). Clin Lymphoma Myeloma Leuk. 2021;21(12):e985–99. https://doi.org/10.1016/j.clml.2021.07.022.
UK Cll Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–72. https://doi.org/10.3324/haematol.2016.147900.
Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020;35(1):107–14. https://doi.org/10.1093/heapol/czz127.
Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials: extrapolation with patient-level data. 2011. https://www.ncbi.nlm.nih.gov/books/NBK395885/pdf/Bookshelf_NBK395885.pdf. Accessed 13 Aug 2023.
Mitchell M. Engauge digitizer software. http://markummitchell.github.io/engauge-digitizer. Accessed 28 Dec 2023.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. https://doi.org/10.1186/1471-2288-12-9.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 28 Dec 2023.
Social Security. Actuarial life table. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed 23 Aug 2023.
Lexicomp. https://www.wolterskluwer.com/en/solutions/lexicomp/lexicomp. Accessed 23 Apr 2023.
Alrawashdh N, McBride A, Erstad B, Sweasy J, Persky DO, Abraham I. Cost-effectiveness and economic burden analyses on all first-line treatments of chronic lymphocytic leukemia. Value Health. 2022;25(10):1685–95. https://doi.org/10.1016/j.jval.2022.04.001.
Reyes C, Engel-Nitz NM, DaCosta BS, Ravelo A, Ogale S, Bancroft T, et al. Cost of disease progression in patients with chronic lymphocytic leukemia, acute myeloid leukemia, and non-Hodgkin’s lymphoma. Oncologist. 2019;24(9):1219–28. https://doi.org/10.1634/theoncologist.2018-0019.
Munir T, Genovez V, Genestier V, Ryan K, Liljas B, Gaitonde P. Cost-effectiveness of acalabrutinib regimens in treatment-naïve chronic lymphocytic leukemia in the United States. Expert Rev Pharmacoecon Outcomes Res. 2023;23(5):579–89. https://doi.org/10.1080/14737167.2023.2196408.
National Cancer Institute. Financial burden of cancer care. https://progressreport.cancer.gov/after/economic_burden. Accessed 25 Aug 2023.
Federal Reserve Economic Data. Consumer price index for all urban consumers: medical care in U.S. City average. https://fred.stlouisfed.org/series/CPIMEDSL. Accessed 23 Aug 2023.
National Institute for Health and Care Excellence. Venetoclax with rituximab for previously treated chronic lymphocytic leukaemia. 2019. http://www.nice.org.uk/guidance/ta561. Accessed 29 Nov 2023.
Patel KK, Isufi I, Kothari S, Davidoff AJ, Gross CP, Huntington SF. Cost-effectiveness of first-line vs third-line ibrutinib in patients with untreated chronic lymphocytic leukemia. Blood. 2020;136(17):1946–55. https://doi.org/10.1182/blood.2020004922.
Barnes JI, Divi V, Begaye A, Wong R, Coutre S, Owens DK, et al. Cost-effectiveness of ibrutinib as first-line therapy for chronic lymphocytic leukemia in older adults without deletion 17p. Blood Adv. 2018;2(15):1946–56. https://doi.org/10.1182/bloodadvances.2017015461.
Neumann PJ, Kim DD. Cost-effectiveness thresholds used by study authors, 1990–2021. JAMA. 2023;329(15):1312–4. https://doi.org/10.1001/jama.2023.1792.
Hatswell AJ, Bullement A, Briggs A, Paulden M, Stevenson MD. Probabilistic sensitivity analysis in cost-effectiveness models: determining model convergence in cohort models. Pharmacoeconomics. 2018;36(12):1421–6. https://doi.org/10.1007/s40273-018-0697-3.
National Cancer Institute. Cancer Stat Facts: leukemia: chronic lymphocytic leukemia (CLL). https://seer.cancer.gov/statfacts/html/clyl.html. Accessed 28 Nov 2023.
Barbier M, Durno N, Bennison C, Örtli M, Knapp C, Schwenkglenks M. Cost-effectiveness and budget impact of venetoclax in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia in Switzerland. Eur J Health Econ. 2022;23(5):837–46. https://doi.org/10.1007/s10198-021-01398-7.
Vemer P, Corro RI, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61. https://doi.org/10.1007/s40273-015-0327-2.
Vreman RA, Geenen JW, Hövels AM, Goettsch WG, Leufkens HGM, Al MJ. Phase I/II clinical trial-based early economic evaluation of acalabrutinib for relapsed chronic lymphocytic leukaemia. Appl Health Econ Health Policy. 2019;17(6):883–93. https://doi.org/10.1007/s40258-019-00496-1.
Cull G, Burger JA, Opat S, Gottlieb D, Verner E, Trotman J, et al. Zanubrutinib for treatment-naïve and relapsed/refractory chronic lymphocytic leukaemia: long-term follow-up of the phase I/II AU-003 study. Br J Haematol. 2022;196(5):1209–18. https://doi.org/10.1111/bjh.17994.
Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23. https://doi.org/10.1056/NEJMoa1400376.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–28. https://doi.org/10.1056/NEJMoa1812836.
Tai TA, Latimer NR, Benedict Á, Kiss Z, Nikolaou A. Prevalence of immature survival data for anti-cancer drugs presented to the National Institute for Health and Care Excellence and impact on decision making. Value Health. 2021;24(4):505–12. https://doi.org/10.1016/j.jval.2020.10.016.
Leighl NB, Nirmalakumar S, Ezeife DA, Gyawali B. An arm and a leg: the rising cost of cancer drugs and impact on access. Am Soc Clin Oncol Educ Book. 2021;41:1–12. https://doi.org/10.1200/EDBK_10002842.
Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35. https://doi.org/10.1016/j.jval.2016.02.017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of interest/competing interests
Dong-Won Kang, Li Wang, Nicholas J. Short, Alessandra Ferrajoli, Yucai Wang, Shouhao Zhou, and Chan Shen have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The ALPINE trial data were obtained from the original article: Brown et al. [8]. Other input data were obtained from original articles and publicly available data. The data are available upon reasonable request.
Code availability
Not applicable.
Authors’ contributions
CS and SZ developed the concept and design of the study. D-WK developed the model and collected and analyzed the data. All authors interpreted the data. D-WK and CS wrote the first draft. All authors contributed to the critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. CS and SZ supervised the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, DW., Wang, L., Short, N.J. et al. Cost Effectiveness of Zanubrutinib Versus Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. PharmacoEconomics 42, 409–418 (2024). https://doi.org/10.1007/s40273-023-01346-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01346-8