Abstract
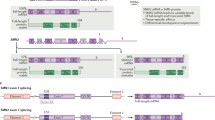
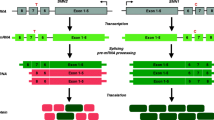
Spinal muscular atrophy (SMA) is an autosomal recessive degenerative neuromuscular disorder characterized by loss of spinal motor neurons leading to muscle weakness and atrophy that is caused by survival motor neuron (SMN) protein deficiency resulting from the biallelic loss of the SMN1 gene. The SMN2 gene modulates the SMA phenotype, as a small fraction of its transcripts are alternatively spliced to produce full-length SMN (fSMN) protein. SMN-targeted therapies increase SMN protein; mRNA therapies, nusinersen and risdiplam, increase the amount of fSMN transcripts alternatively spliced from the SMN2 gene, while gene transfer therapy, onasemnogene abeparvovec xioi, increases SMN protein by introducing the hSMN gene into various tissues, including spinal cord via an AAV9 vector. These SMN-targeted therapies have been found effective in improving outcomes and are approved for use in SMA in the US and elsewhere. This article discusses the clinical trial results for SMN-directed therapies with a focus on efficacy, side effects and treatment response predictors. It also discusses preliminary data from muscle-targeted trials, as single agents and in combination with SMN-targeted therapies, as well as other classes of SMA treatments.


Similar content being viewed by others
References
Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65.
Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16(3):265–9.
Sugarman EA, Nagan N, Zhu H, Akmaev VR, Zhou Z, Rohlfs EM, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. 2012;20(1):27–32.
Vill K, Kölbel H, Schwartz O, Blaschek A, Olgemöller B, Harms E, et al. One year of newborn screening for SMA—results of a German Pilot Project. J Neuromuscul Dis. 2019;6(4):503–15.
Kay DM, Stevens CF, Parker A, Saavedra-Matiz CA, Sack V, Chung WK, et al. Implementation of population-based newborn screening reveals low incidence of spinal muscular atrophy. Genet Med. 2020;22(8):1296–302.
Calucho M, Bernal S, Alías L, March F, Venceslá A, Rodríguez-Álvarez FJ, et al. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. 2018;28(3):208–15.
Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883–91.
Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–7.
Glanzman AM, McDermott MP, Montes J, Martens WB, Flickinger J, Riley S, et al. Validation of the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND). Pediatr Phys Ther. 2011;23(4):322–6.
Bayley N. Bayley Scales of Infant Development. 3rd ed. Coushatta: Pearson Assessments; 2005.
Haataja L, Mercuri E, Regev R, Cowan F, Rutherford M, Dubowitz V, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135(2 Pt 1):153–61.
WHO. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450:86–95.
Kaufmann P, McDermott MP, Darras BT, Finkel R, Kang P, Oskoui M, et al. Observational study of spinal muscular atrophy type 2 and 3: functional outcomes over 1 year. Arch Neurol. 2011;68(6):779–86.
Mercuri E, Lucibello S, Pera MC, Carnicella S, Coratti G, de Sanctis R, et al. Long-term progression in type II spinal muscular atrophy: a retrospective observational study. Neurology. 2019;93(13):e1241–7.
O’Hagen JM, Glanzman AM, McDermott MP, Ryan PA, Flickinger J, Quigley J, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. 2007;17(9–10):693–7.
Vuillerot C, Payan C, Girardot F, Fermanian J, Iwaz J, Berard C, et al. Responsiveness of the motor function measure in neuromuscular diseases. Arch Phys Med Rehabil. 2012;93(12):2251-2256 e1.
Mazzone ES, Mayhew A, Montes J, Ramsey D, Fanelli L, Young SD, et al. Revised upper limb module for spinal muscular atrophy: development of a new module. Muscle Nerve. 2017;55(6):869–74.
Zerres K, Rudnik-Schoneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa-Petrusewicz I. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci. 1997;146(1):67–72.
Lin CW, Kalb SJ, Yeh WS. Delay in diagnosis of spinal muscular atrophy: a systematic literature review. Pediatr Neurol. 2015;53(4):293–300.
Montes J, McDermott MP, Martens WB, Dunaway S, Glanzman AM, Riley S, et al. Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010;74(10):833–8.
Wirth B, Herz M, Wetter A, Moskau S, Hahnen E, Rudnik-Schoneborn S, et al. Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am J Hum Genet. 1999;64(5):1340–56.
Burghes AH, Beattie CE, Burghes AHM, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10(8):597–609.
Pellizzoni L, Yong J, Dreyfuss G. Essential role for the SMN complex in the specificity of snRNP assembly. Science. 2002;298(5599):1775–9.
Gabanella F, Carissimi C, Usiello A, Pellizzoni L. The activity of the spinal muscular atrophy protein is regulated during development and cellular differentiation. Hum Mol Genet. 2005;14(23):3629–42.
Ramos DM, d’Ydewalle C, Gabbeta V, Dakka A, Klein SK, Norris DA, et al. Age-dependent SMN expression in disease-relevant tissue and implications for SMA treatment. J Clin Investig. 2019;129(11):4817–31.
Le TT, McGovern VL, Alwine IE, Wang X, Massoni-Laporte A, Rich MM, et al. Temporal requirement for high SMN expression in SMA mice. Hum Mol Genet. 2011;20(18):3578–91.
Harding BN, Kariya S, Monani UR, Chung WK, Benton M, Yum SW, et al. Spectrum of neuropathophysiology in spinal muscular atrophy type I. J Neuropathol Exp Neurol. 2015;74(1):15–24.
McGovern VLIC, Arnold WD, Gombash SE, Zaworski PG, Blatnik AJ 3rd, et al. SMN expression is required in motor neurons to rescue electrophysiological deficits in the SMNΔ7 mouse model of SMA. Hum Mol Genet. 2015;24(19):27.
Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, et al. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69(3):453–67.
Imlach WL, Beck ES, Choi BJ, Lotti F, Pellizzoni L, McCabe BD. SMN is required for sensory-motor circuit function in Drosophila. Cell. 2012;151(2):427–39.
Singh RN, Howell MD, Ottesen EW, Singh NN. Diverse role of survival motor neuron protein. Biochim Biophys Acta. 2017;1860(3):299–315.
Rudnik-Schöneborn S, Heller R, Berg C, Betzler C, Grimm T, Eggermann T, et al. Congenital heart disease is a feature of severe infantile spinal muscular atrophy. J Med Genet. 2008;45(10):635–8.
Rudnik-Schöneborn S, Vogelgesang S, Armbrust S, Graul-Neumann L, Fusch C, Zerres K. Digital necroses and vascular thrombosis in severe spinal muscular atrophy. Muscle Nerve. 2010;42(1):144–7.
Araujo A, Araujo M, Swoboda KJ. Vascular perfusion abnormalities in infants with spinal muscular atrophy. J Pediatr. 2009;155(2):292–4.
Tein I, Sloane AE, Donner EJ, Lehotay DC, Millington DS, Kelley RI. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatr Neurol. 1995;12(1):21–30.
Crawford TO, Sladky JT, Hurko O, Besner-Johnston A, Kelley RI. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann Neurol. 1999;45(3):337–43.
Hachiya Y, Arai H, Hayashi M, Kumada S, Furushima W, Ohtsuka E, et al. Autonomic dysfunction in cases of spinal muscular atrophy type 1 with long survival. Brain Dev. 2005;27(8):574–8.
Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103–15.
Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum Mol Genet. 2010;19(20):4059–71.
Gombash SE, Cowley CJ, Fitzgerald JA, Iyer CC, Fried D, McGovern VL, et al. SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum Mol Genet. 2015;24(19):5665.
Bowerman M, Swoboda KJ, Michalski JP, Wang GS, Reeks C, Beauvais A, et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol. 2012;72(2):256–68.
Mutsaers CA, Wishart TM, Lamont DJ, Riessland M, Schreml J, Comley LH, et al. Reversible molecular pathology of skeletal muscle in spinal muscular atrophy. Hum Mol Genet. 2011;20(22):4334–44.
Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA. 1999;96(11):6307–11.
Mailman MD, Heinz JW, Papp AC, et al. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet Med. 2002;4(1):20–6.
Crawford TO, Paushkin SV, Kobayashi DT, et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS One. 2012;7(4): e33572.
Prior TW, Krainer AR, Hua Y, Swoboda KJ, Snyder PC, Bridgeman SJ, et al. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am J Hum Genet. 2009;85(3):408–13.
Vezain M, Saugier-Veber P, Goina E, Touraine R, Manel V, Toutain A, et al. A rare SMN2 variant in a previously unrecognized composite splicing regulatory element induces exon 7 inclusion and reduces the clinical severity of spinal muscular atrophy. Hum Mutat. 2010;31(1):E1110–25.
Wu X, Wang SH, Sun J, Krainer AR, Hua Y, Prior TW. A-44G transition in SMN2 intron 6 protects patients with spinal muscular atrophy. Hum Mol Genet. 2017;26(14):2768–80.
Ruhno C, McGovern VL, Avenarius MR, Snyder PJ, Prior TW, Nery FC, et al. Complete sequencing of the SMN2 gene in SMA patients detects SMN gene deletion junctions and variants in SMN2 that modify the SMA phenotype. Hum Genet. 2019;138(3):241–56.
FDA. Spinraza (nusinersen) product label: Reference ID: 4625921—December 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/209531lbl.pdf (Accessed 15 Feb 2022)
EMA. Summary of opinion (initial authorization) Spinraza nusinersen 2017 April 21. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-spinraza_en.pdf.
FDA. BLA Clinical Review Memorandum (ZOLGENSMA, onasemnogene abeparvovec xioi); 2019 May 23. https://www.fda.gov/vaccines-blood-biologics/zolgensma (Accessed 15 Feb 2022).
EMA. Zolgensma (onasemnogene abeparvovec) [updated 6/12/2021]; 2020 March 26. https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma (Accessed Oct 2020).
FDA. Center for Drug Evaluation and Research. Application number: 213535Orig1s00. Clinical Reviews. EVRYSDI (risdiplam). 2020 June 30. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213535Orig1s000MedR.pdf (Accessed 15 Feb 2022).
EMA. Orphan Maintenance Assessment report-Evrysdi (risdiplam). 2021 March 26. https://www.ema.europa.eu/en/documents/orphan-maintenance-report/evrysdi-orphan-maintenance-assessment-report-initial-authorisation_en.pdf (Accessed 20 June 2022).
Genentech. FDA approves Genentech’s Evrysdi (risdiplam) for use in babies under two months with spinal muscular atrophy (SMA); 2022 May 30. https://www.gene.com/media/press-releases/14955/2022-05-30/fda-approves-genentechs-evrysdi-risdiplam (Accessed 6 July 2022).
Singh NK, Singh NN, Androphy EJ, Singh RN. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol Cell Biol. 2006;26(4):1333–46.
Hua Y, Vickers TA, Okunola HL, Bennett CF, Krainer AR. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet. 2008;82:834–48.
Passini MA, Bu J, Richards AM, Kinnecom C, Sardi SP, Stanek LM, et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med. 2011;2(3):11.
Finkel RSME, Darras B, Connolly A, Kunz N, Kirschner J, et al. For the ENDEAR Study Group efficacy and safety of nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–32.
FDA. Center for Drug Evaluation and Research. Application number: 209531Orig1s000. Medical Reviews. Spinraza (nusinersen); December 15, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/209531Orig1s000MedR.pdf (Accessed March 2017).
Finkel RS, Chiriboga CA, Vajsar J, Day J, Montes J, De Vivo DC, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:10063.
Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, De Vivo DC, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc Health. 2021;5(7):491–500.
Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625–35.
Chiriboga CA, Darras BT, Farrar MA, Mercuri E, Kirschner J, Kuntz NL, et al. Interim report on the safety and efficacy of longer-term treatment with nusinersen in later-onset spinal muscular atrophy (SMA): results from the SHINE study. 48th Child Neurology Society Meeting 2019 October 23–29, Charlotte.
Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, De Vivo DC, et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology. 2016;86(10):890–7.
Darras BT, Chiriboga CA, Iannaccone ST, Swoboda KJ, Montes J, Mignon L, et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the phase 1/2 studies. Neurology. 2019;92(21):e2492–506.
De Vivo DC, Bertini E, Swoboda KJ, Hwu WL, Crawford TO, Finkel RS, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842–56.
Sansone V SK, DeVivo DC, Bertini E, Hwu WL, Makepeace C, et al. Preserved swallowing function in Infants who Initiated nusinersen treatment in the presyptomatic stage of SMA: results from the NURTURE study World Muscle Society 26th International Congress; Virtual 2021 Sep 20–24.
Hagenacker T, Wurster CD, Günther R, Schreiber-Katz O, Osmanovic A, Petri S, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19(4):317–25.
Maggi L, Bello L, Bonanno S, Govoni A, Caponnetto C, Passamano L, et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry. 2020;91(11):1166–74.
Elsheikh B, Severyn S, Zhao S, Kline D, Linsenmayer M, Kelly K, et al. Safety, tolerability, and effect of nusinersen treatment in ambulatory adults with 5q-SMA. Front Neurol. 2021;12: 650535.
Walter MC, Wenninger S, Thiele S, Stauber J, Hiebeler M, Greckl E, et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3—a prospective observational study. J Neuromuscul Dis. 2019;6(4):453–65.
Biogen. Spinraza (nusinersen): reports of communicating hydrocephalus not related to meningitis or bleeding; 2018 July 31. https://assets.publishing.service.gov.uk/media/5b7edf83ed915d14f4404bf6/Spinraza_UK_DHPC_SPZ_GBR_0020.pdf (Accessed 15 Feb 2022).
Viscidi E, Wang N, Juneja M, Bhan I, Prada C, James D, et al. The incidence of hydrocephalus among patients with and without spinal muscular atrophy (SMA): results from a US electronic health records study. Orphanet J Rare Dis. 2021;16(1):207.
Moshe-Lilie O, Riccelli LP, Karam C. Possible recurrent aseptic meningitis associated with nusinersen therapy. Muscle Nerve. 2020;62(5):E79–80.
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–80.
Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271–4.
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65.
Duque SI, Arnold WD, Odermatt P, Li X, Porensky PN, Schmelzer L, et al. A large animal model of spinal muscular atrophy and correction of phenotype. Ann Neurol. 2015;77(3):399–414.
Thomsen G, Burghes AHM, Hsieh C, Do J, Chu BTT, Perry S, et al. Biodistribution of onasemnogene abeparvovec DNA, mRNA and SMN protein in human tissue. Nat Med. 2021;27(10):1701–11.
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–22.
Lowes LP, Alfano LN, Arnold WD, Shell R, Prior TW, McColly M, et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol. 2019;98:39–45.
Day JW, Finkel RS, Chiriboga CA, Connolly AM, Crawford TO, Darras BT, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–93.
Mercuri E, Muntoni F, Baranello G, Masson R, Boespflug-Tanguy O, Bruno C, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(10):832–41.
Strauss KA FM, Swoboda KJ, Saito K, Chiriboga CA, Finkel RS, et al. Onasemnogene abeparvovec in presymptomatic spinal muscular atrophy: SPR1NT study update as of 31 Dec 2019. Muscular Dystrophy Association (MDA) Clinical and Scientific Meeting Virtual; 2020 March 21–24.
Strauss KA MF, Farra MA, Saito K, Mendell J, Servais L, et al. Abeparvovec in presymptomatic spinal muscular atrophy: SPR1NT study update in children with 2 copies of SMN2. American Academy of Neurology 73rd Annual Meeting; 2021 April 17.
Chand D, Mohr F, McMillan H, Tukov FF, Montgomery K, Kleyn A, et al. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J Hepatol. 2021;74(3):560–6.
Chand DH, Zaidman C, Arya K, Millner R, Farrar MA, Mackie FE, et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J Pediatr. 2021;231:265–8.
Novartis. Novartis announces lift of partial clinical trial hold and plans to initiate a new, pivotal Phase 3 study of intrathecal OAV-101 in older patients with SMA. News release. August 3, 2021. https://www.novartis.com/news/media-releases/novartis-announces-lift-partial-clinical-trial-hold-and-plans-initiate-new-pivotal-phase-3-study-intrathecal-oav-101-older-patients-sma (Accessed 15 Feb 2022).
Palacino J, Swalley SE, Song C, Cheung AK, Shu L, Zhang X, et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat Chem Biol. 2015;11(7):511–7.
Naryshkin NA, Weetall M, Dakka A, Narasimhan J, Zhao X, Feng Z, et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345(6197):688–93.
Ratni H, Ebeling M, Baird J, Bendels S, Bylund J, Chen KS, et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA). J Med Chem. 2018;61(15):6501–17.
Sergott RC, Amorelli GM, Baranello G, Barreau E, Beres S, Kane S, et al. Risdiplam treatment has not led to retinal toxicity in patients with spinal muscular atrophy. Ann Clin Transl Neurol. 2021;8(1):54–65.
Poirier A, Weetall M, Heinig K, Bucheli F, Schoenlein K, Alsenz J, et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol Res Perspect. 2018;6(6): e00447.
Baranello G, Darras BT, Day JW, Deconinck N, Klein A, Masson R, et al. Risdiplam in type 1 spinal muscular atrophy. N Engl J Med. 2021;384(10):915–23.
Mercuri E, Deconinck N, Mazzone ES, Nascimento A, Oskoui M, Saito K, et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022;21(1):42–52.
Chiriboga CA, Bruno CDT, Fischer D, Kirschner J, Mercuri E, et al. JEWELFISH: safety, pharmacodynamic and exploratory efficacy data in non-naïve patients with SMA receiving treatment with risdiplam. MDA Clinical & Scientific Conference, Nashville, 13–16 March 2022.
Servais L, Al-Muhaizea M, Farrar M, Nelson L, Prufer A, Finkel R, et al. RAINBOWFISH: a study of risdiplam in infants with presymptomatic spinal muscular atrophy (SMA). Neuromuscul Disord J. 2021;31:S48.
Svetlana J CD, Dobrzycka-Ambrozewicz A, Kotulscka-Jozwiak K, Lvova O, Pervinina Y, et al. Branaplam in type 1 spinal muscular atrophy: second and third parts of a phase I/II study 26th World Muscle Society Virtual Annual Congress, 20–24 September 2021.
Sumner CJ, Crawford TO. Two breakthrough gene-targeted treatments for spinal muscular atrophy: challenges remain. J Clin Investig. 2018;128(8):3219–27.
Osmanovic A, Schreiber-Katz O, Petri S. Nusinersen wearing-off in adult 5q-spinal muscular atrophy patients. Brain Sci. 2021;11(3):367.
Mercuri EFR, Day JW, Pascual Pascual PI, Ryan MM, DeVivo DC, et al. editors. Part A result from ongoing DEVOTE study to explore higher doses of nusinersen in SMA. World Muscle Society 26th International Virtual Congress, 20–24 Sep 2021.
Mendell JR, Al-Zaidy SA, Lehman KJ, McColly M, Lowes LP, Alfano LN, et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78(7):834–41.
Long KK, O’Shea KM, Khairallah RJ, Howell K, Paushkin S, Chen KS, et al. Specific inhibition of myostatin activation is beneficial in mouse models of SMA therapy. Hum Mol Genet. 2019;28(7):1076–89.
Cure SMA. Scholar rock presents TOPAZ phase 2 data showing the transformative potential of apitegromab in patients with type 2 and 3 SMA; 2021 June 11. https://www.curesma.org/scholar-rock-topaz-data-for-apitegromab-researcher-meeting-2021/ (Accessed 15 Feb 2022).
Rudnicki SA, Andrews JA, Duong T, Cockroft BM, Malik FI, Meng L, et al. Reldesemtiv in patients with spinal muscular atrophy: a phase 2 hypothesis-generating study. Neurotherapeutics. 2021;18(2):1127–36.
Brichta L, Hofmann Y, Hahnen E, Siebzehnrubl FA, Raschke H, Blumcke I, et al. Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet. 2003;12(19):2481–9.
Swoboda KJ, Scott CB, Crawford TO, Simard LR, Reyna SP, Krosschell KJ, et al. SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS One. 2010;5(8): e12140.
Kissel JT, Scott CB, Reyna SP, Crawford TO, Simard LR, Krosschell KJ, et al. SMA CARNIVAL TRIAL PART II: a prospective, single-armed trial of l-carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PLoS One. 2011;6(7): e21296.
Mercuri E, Bertini E, Messina S, Solari A, D’Amico A, Angelozzi C, et al. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology. 2007;68(1):51–5.
Chen TH, Chang JG, Yang YH, Mai HH, Liang WC, Wu YC, et al. Randomized, double-blind, placebo-controlled trial of hydroxyurea in spinal muscular atrophy. Neurology. 2010;75(24):2190–7.
Garbes L, Riessland M, Hölker I, Heller R, Hauke J, Tränkle C, et al. LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate. Hum Mol Genet. 2009;18(19):3645–58.
Pagliarini V, Guerra M, Di Rosa V, Compagnucci C, Sette C. Combined treatment with the histone deacetylase inhibitor LBH589 and a splice-switch antisense oligonucleotide enhances SMN2 splicing and SMN expression in spinal muscular atrophy cells. J Neurochem. 2020;153(2):264–75.
Bartels B, de Groot JF, Habets LE, Wadman RI, Asselman FL, Nieuwenhuis EES, et al. Correlates of fatigability in patients with spinal muscular atrophy. Neurology. 2021;96(6):e845–52.
Kariya S, Park GH, Maeno-Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS, et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet. 2008;17(16):2552–69.
Wadman RI, Vrancken AF, van den Berg LH, van der Pol WL. Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Neurology. 2012;79(20):2050–5.
Pera MC, Luigetti M, Pane M, Coratti G, Forcina N, Fanelli L, et al. 6MWT can identify type 3 SMA patients with neuromuscular junction dysfunction. Neuromuscul Disord. 2017;27(10):879–82.
Chiriboga CA, Marra J, LaMarca NM, Young SD, Weimer LH, Levin B, et al. Lack of effect on ambulation of dalfampridine-ER (4-AP) treatment in adult SMA patients. Neuromuscul Disord. 2020;30(8):693–700.
Stam M, Wadman RI, Wijngaarde CA, Bartels B, Asselman FL, Otto LAM, et al. Protocol for a phase II, monocentre, double-blind, placebo-controlled, cross-over trial to assess efficacy of pyridostigmine in patients with spinal muscular atrophy types 2–4 (SPACE trial). BMJ Open. 2018;8(7): e019932.
Tiziano FD, Lomastro R, Pinto AM, Messina S, D’Amico A, Fiori S, et al. Salbutamol increases survival motor neuron (SMN) transcript levels in leucocytes of spinal muscular atrophy (SMA) patients: relevance for clinical trial design. J Med Genet. 2010;47(12):856–8.
Angelozzi C, Borgo F, Tiziano FD, Martella A, Neri G, Brahe C. Salbutamol increases SMN mRNA and protein levels in spinal muscular atrophy cells. J Med Genet. 2008;45(1):29–31.
Pane M, Staccioli S, Messina S, D’Amico A, Pelliccioni M, Mazzone ES, et al. Daily salbutamol in young patients with SMA type II. Neuromuscul Disord. 2008;18(7):536–40.
Frongia AL, Natera-de Benito D, Ortez C, Alarcón M, Borrás A, Medina J, et al. Salbutamol tolerability and efficacy in patients with spinal muscular atrophy type II. Neuromuscul Disord. 2019;29(7):517–24.
Tiziano FD, Lomastro R, Abiusi E, Pasanisi MB, Di Pietro L, Fiori S, et al. Longitudinal evaluation of SMN levels as biomarker for spinal muscular atrophy: results of a phase IIb double-blind study of salbutamol. J Med Genet. 2019;56(5):293–300.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was provided for the writing of this manuscript.
Conflict of interest
Dr. Chiriboga receives grant funding from Avexis/Novartis, Biogen and Roche. She serves/served as consultant for Roche, Genentech, Avexis/Novartis and PTC and has served as Speaker in unlabeled educational talks for Biogen, Roche and Genentech.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Dr. Chiriboga was the sole author of this article.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiriboga, C.A. Pharmacotherapy for Spinal Muscular Atrophy in Babies and Children: A Review of Approved and Experimental Therapies. Pediatr Drugs 24, 585–602 (2022). https://doi.org/10.1007/s40272-022-00529-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00529-8