Abstract
Background and Objectives
Despite the evidence that no other antipsychotic is effective as clozapine for the treatment of resistant schizophrenia, it is associated with various metabolic, neuroendocrine, cardiovascular, and gastrointestinal adverse effects. Guidelines aiming to address the monitoring of clozapine’s (serious) adverse effects can be helpful to prevent and treat these effects. However, many of these guidelines seem to lack one or more important monitoring recommendations. We aimed to systematically review the content and quality of existing monitoring guidelines/recommendations for clozapine-induced adverse effects.
Methods
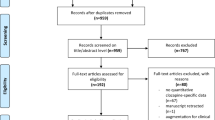
A comprehensive and systematic literature search, using the MEDLINE, Embase, Web of Science, and Cochrane databases, was conducted for guidelines/recommendations on the monitoring of clozapine-induced adverse events, published between January 2004 and April 2023 (last search 16 April 2023). Only peer-reviewed published guidelines reporting on the comprehensive monitoring of all major clozapine-induced adverse effects and including evidence-based recommendations, developed after the year 2004, were included. Studies reporting on the monitoring of adverse effects of clozapine without being a formal guideline, guidelines reporting on the monitoring of one or a limited number of adverse effects of clozapine, guidelines that were not peer reviewed or published, expert opinion papers without formal consensus guideline development, or guidelines developed before the year 2004, were excluded. The Appraisal of Guidelines for Research and Evaluation II (AGREE-II) tool was used to evaluate the guidelines/recommendations’ quality.
Results
Only one guideline met the inclusion criteria. This consensus statement made recommendations for hematological monitoring, and the monitoring of metabolic, cardiac, and three other adverse effects. Highest scores for the qualitative assessment were found for the domains “scope and purpose” (66.7%), “clarity of presentation” (44.4%), and “editorial independence” (66.7%). Lowest scores were found for “rigor of development” (14.6%) and “applicability” (0%).
Conclusions
Future guidelines should develop more comprehensive recommendations about specific clozapine-induced adverse effects, including constipation, myocarditis, tachycardia, and seizures, as well as include a rechallenge policy. There is an urgent need for well-developed, methodologically stringent, guidelines.
Registration
PROSPERO registration number, CRD42023402480.

Similar content being viewed by others
References
World Health Organization. Mental health: schizophrenia. World Health Organization. 2022. https://www.who.int/news-room/fact-sheets/detail/schizophrenia. Accessed 24 Apr 2023.
Siskind D, Orr S, Sinha S, Yu O, Brijball B, Warren N, et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br J Psychiatry. 2022;220(3):115–20. https://doi.org/10.1192/bjp.2021.61.
Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29(2):63–76. https://doi.org/10.1097/YIC.0b013e32836508e6.
Masdrakis VG, Baldwin DS. Prevention of suicide by clozapine in mental disorders: systematic review. Eur Neuropsychopharmacol. 2023;69:4–23. https://doi.org/10.1016/j.euroneuro.2022.12.011.
Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–96. https://doi.org/10.1001/archpsyc.1988.01800330013001.
Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772–7. https://doi.org/10.1177/0706743717718167.
Vanasse A, Blais L, Courteau J, Cohen AA, Roberge P, Larouche A, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374–84. https://doi.org/10.1111/acps.12621.
Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am Journal of Psychiatry. 2016;173(2):166–73. https://doi.org/10.1176/appi.ajp.2015.15030332.
Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiat. 2017;74(7):686–93. https://doi.org/10.1001/jamapsychiatry.2017.1322.
Dong S, Schneider-Thoma J, Bighelli I, Siafis S, Wang D, Burschinski A, et al. A network meta-analysis of efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023. https://doi.org/10.1007/s00406-023-01654-2.
Psychosis and schizophrenia in adults: prevention and management. National Institute for Health and Care Excellence: Guidelines. London: National Institute for Health and Care Excellence (NICE); 2014.
Barnes TRE, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2019;34(1):3–78. https://doi.org/10.1177/0269881119889296.
Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aus N Z J Psychiatry. 2016;50(5):410–72. https://doi.org/10.1177/0004867416641195.
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2–44. https://doi.org/10.3109/15622975.2012.739708.
Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, Servis M, Walaszek A, Buckley P, Lenzenweger MF, Young AS, Degenhardt A, Hong S. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177(9):868–72. https://doi.org/10.1176/appi.ajp.2020.177901.
Avasthi A, Sahoo S, Grover S. Clinical practice guidelines for cognitive behavioral therapy for psychotic disorders. Indian J Psychiatry. 2020;62(8):S251–62. https://doi.org/10.4103/psychiatry.IndianJPsychiatry_774_19.
Lee JS, Yun J-Y, Kang SH, Lee SJ, Choi J-H, Nam B, et al. Korean medication algorithm for schizophrenia 2019, second revision: treatment of psychotic symptoms. Clin Psychopharmacol Neurosci. 2020;18(3):386–94. https://doi.org/10.9758/cpn.2020.18.3.386.
Noel JM, Jackson CW. ASHP therapeutic position statement on the use of antipsychotic medications in the treatment of adults with schizophrenia and schizoaffective disorder. Am J Health Syst Pharm. 2020;77(24):2114–32. https://doi.org/10.1093/ajhp/zxaa303.
Osser DN, Roudsari MJ, Manschreck T. The psychopharmacology algorithm project at the Harvard South Shore Program: an update on schizophrenia. Harv Rev Psychiatry. 2013;21(1):18–40. https://doi.org/10.1097/HRP.0b013e31827fd915.
Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62(9):604–16. https://doi.org/10.1177/0706743717720448.
Network SIG. Management of schizophrenia: a national clinical guideline: Scottish Intercollegiate Guidelines Network; 2013.
Stahl SM, Morrissette DA, Citrome L, Saklad SR, Cummings MA, Meyer JM, et al. “Meta-guidelines” for the management of patients with schizophrenia. CNS Spectr. 2013;18(3):150–62. https://doi.org/10.1017/S109285291300014X.
Taylor DM, Barnes TR, Young AH. The Maudsley prescribing guidelines in psychiatry. 14th ed. Wiley; 2021.
Verma S, Chan LL, Chee KS, Chen H, Chin SA, Chong SA, et al. Ministry of Health clinical practice guidelines: schizophrenia. Singap Med J. 2011;52(7):521–5 (quiz 6).
Sakurai H, Yasui-Furukori N, Suzuki T, Uchida H, Baba H, Watanabe K, et al. Pharmacological treatment of schizophrenia: Japanese expert consensus. Pharmacopsychiatry. 2021;54(02):60–7.
Citrome L, McEvoy JP, Saklad SR. Guide to the management of clozapine-related tolerability and safety concerns. Clin Schizophr Relat Psychoses. 2016;10(3):163–77. https://doi.org/10.3371/1935-1232.10.3.163.
Lee MA, Cola P, Jayathilake K, Meltzer HY. Long-term outcome of clozapine in treatment-resistant schizophrenia. J Clinl Psychopharmacol. 2023;43(3):211–9. https://doi.org/10.1097/jcp.0000000000001671.
Nielsen J, Dahm M, Lublin H, Taylor D. Psychiatrists’ attitude towards and knowledge of clozapine treatment. J Psychopharmacol. 2010;24(7):965–71. https://doi.org/10.1177/0269881108100320.
Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136(1):37–51. https://doi.org/10.1111/acps.12742.
Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481–5. https://doi.org/10.1192/bjp.bp.111.105833.
Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224–7. https://doi.org/10.1176/appi.ps.201700162.
Farooq S, Choudry A, Cohen D, Naeem F, Ayub M. Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. BJ Psych Bull. 2019;43(1):8–16. https://doi.org/10.1192/bjb.2018.67.
Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53–8. https://doi.org/10.1007/s40596-019-01134-7.
Oloyede E, Blackman G, Mantell B, Harris E, Williams J, Taylor D, et al. What are the barriers and facilitators of clozapine use in early psychosis? A survey of UK early intervention clinicians. Schizophrenia. 2023;9(1):26. https://doi.org/10.1038/s41537-023-00353-0.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160. https://doi.org/10.1136/bmj.n160.
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267–72.
AGREE Next Steps Consortium. The AGREE II Instrument [Electronic version]. AGREE Next Steps Consortium. 2017. http://www.agreetrust.org. Accessed 8 May 2023.
Berk M, Fitzsimons J, Lambert T, Pantelis C, Kulkarni J, Castle D, et al. Monitoring the safe use of clozapine: a consensus view from Victoria. Australia CNS Drugs. 2007;21(2):117–27. https://doi.org/10.2165/00023210-200721020-00003.
World Health Organization. Web Annex A. World Health Organization Model List of Essential Medicines—23rd List, 2023. In: The selection and use of essential medicines 2023: Executive summary of the report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines, 24–28 April 2023. Geneva: World Health Organization. 2023. https://iris.who.int/bitstream/handle/10665/371291/WHO-MHP-HPS-EML-2023.01-eng.pdf?sequence=1. Accessed 20 Aug 2023.
Correll CU, Agid O, Crespo-Facorro B, de Bartolomeis A, Fagiolini A, Seppälä N, et al. A guideline and checklist for initiating and managing clozapine treatment in patients with treatment-resistant schizophrenia. CNS Drugs. 2022;36(7):659–79. https://doi.org/10.1007/s40263-022-00932-2.
Cohen D, Bogers JP, van Dijk D, Bakker B, Schulte PF. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307–12. https://doi.org/10.4088/JCP.11r06977.
Wagner E, Siskind D, Falkai P, Howes O, Correll C, Lee J, et al. Clozapine optimization: A Delphi consensus guideline from the Treatment Response and Resistance in Psychosis Working Group. Schizophr Bull. 2023. https://doi.org/10.1093/schbul/sbad030.
Manu P, Sarvaiya N, Rogozea LM, Kane JM, Correll CU. Benign ethnic neutropenia and clozapine use: a systematic review of the evidence and treatment recommendations. J Clin Psychiatry. 2016;77(7):e909–16. https://doi.org/10.4088/JCP.15r10085.
Ronaldson KJ, Taylor AJ, Fitzgerald PB, Topliss DJ, McNeil JJ. New monitoring guidelines for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aus N Z J Psychiatry. 2010;44:A26–7. https://doi.org/10.3109/00048674.2010.507064.
de Leon J, Schoretsanitis G, Smith RL, Molden E, Solismaa A, Seppälä N, et al. An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry. 2022;55(2):73–86. https://doi.org/10.1055/a-1625-6388.
De Hert M, Detraux J, Van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114–26. https://doi.org/10.1038/nrendo.2011.156.
Dutch clozapine collaboration group. Guideline for the use of clozapine. Dutch clozapine collaboration group. 2013. https://www.clozapinepluswerkgroep.nl/wp-content/uploads/2013/07/Guideline-for-the-use-of-Clozapine-2013.pdf. Accessed 31 May 2023.
Association AP. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd ed. Washington, DC: American Psychiatric Association; 2021.
Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophrenia Bull. 2010;36(1):71–93. https://doi.org/10.1093/schbul/sbp116.
Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3–78. https://doi.org/10.1177/0269881119889296.
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–78. https://doi.org/10.3109/15622975.2012.696143.
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia—a short version for primary care. Int J Psychiatry Clin Pract. 2017;21(2):82–90. https://doi.org/10.1080/13651501.2017.1291839.
Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340. https://doi.org/10.1186/1471-244x-13-340.
Japanese Society of Neuropsychopharmacology. Guideline for Pharmacological Therapy of Schizophrenia. Neuropsychopharmacol Rep. 2021;41(3):266–324. https://doi.org/10.1002/npr2.12193.
Leucht S. CINP Schizophrenia Guidelines. The International College of Neuropsychopharmacology. 2013. https://www.cinp.org/resources/Documents/CINP-schizophrenia-guideline-24.5.2013-A-C-method.pdf. Accessed 22 May 2023.
Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601. DOI: https://doi.org/10.2337/diacare.27.2.596
De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199(2):99–105. https://doi.org/10.1192/bjp.bp.110.084665.
De Hert M, Dekker JM, Wood D, Kahl KG, Holt RIG, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24(6):412–24. https://doi.org/10.1016/j.eurpsy.2009.01.005.
Clozapine [package insert]. Sandoz nv/sa. 2020. https://app.fagg-afmps.be/pharma-status/api/files/62bc5d1a1e5c015ab3c8e798. Accessed 19 Aug 2023.
FDA. FDA Drug Safety Communication: FDA modifies monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. Food and Drug Administration. 2015. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-modifies-monitoring-neutropenia-associated-schizophrenia-medicine. Accessed 18 Oct 2023.
Sultan RS, Olfson M, Correll CU, Duncan EJ. Evaluating the effect of the changes in FDA guidelines for clozapine monitoring. J Clin Psychiatry. 2017;78(8):e933–9. https://doi.org/10.4088/JCP.16m11152.
Oloyede E, Blackman G, Whiskey E, Bachmann C, Dzahini O, Shergill S, et al. Clozapine haematological monitoring for neutropenia: a global perspective. Epidemiol Psychiatr Sci. 2022;31: e83. https://doi.org/10.1017/s204579602200066x.
Guideline for the use of clozapine. Dutch clozapine collaboration group. 2013. https://www.clozapinepluswerkgroep.nl/wp-content/uploads/2013/07/Guideline-for-the-use-of-Clozapine-2013.pdf. Accessed 19 Oct 2023.
Tiihonen J, Tanskanen A, Bell JS, Dawson JL, Kataja V, Taipale H. Long-term treatment with clozapine and other antipsychotic drugs and the risk of haematological malignancies in people with schizophrenia: a nationwide case-control and cohort study in Finland. Lancet Psychiatry. 2022;9(5):353–62. https://doi.org/10.1016/S2215-0366(22)00044-X.
Xu Y, Amdanee N, Zhang X. Antipsychotic-induced constipation: a review of the pathogenesis, clinical diagnosis, and treatment. CNS Drugs. 2021;35(12):1265–74. https://doi.org/10.1007/s40263-021-00859-0.
De Hert M, Hudyana H, Dockx L, Bernagie C, Sweers K, Tack J, et al. Second-generation antipsychotics and constipation: a review of the literature. EurPsychiatry. 2011;26(1):34–44. https://doi.org/10.1016/j.eurpsy.2010.03.003.
Chougule A, Praharaj SK, Bhat SM, Sharma P. Prevalence and factors associated with clozapine-related constipation: an observational study. J Clin Psychopharmacol. 2018;38(1):42–6. https://doi.org/10.1097/jcp.0000000000000824.
Every-Palmer S, Inns SJ, Grant E, Ellis PM. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81–91. https://doi.org/10.1007/s40263-018-0587-4.
Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal “slow gut” reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699–709. https://doi.org/10.1007/s40263-017-0448-6.
Every-Palmer S, Newton-Howes G, Clarke MJ. Pharmacological treatment for antipsychotic-related constipation. Cochrane Database System Rev. 2017. https://doi.org/10.1002/14651858.CD011128.pub2.
Every-Palmer S, Ellis PM, Nowitz M, Stanley J, Grant E, Huthwaite M, et al. The Porirua protocol in the treatment of clozapine-induced gastrointestinal hypomotility and constipation: a pre- and post-treatment study. CNS Drugs. 2017;31(1):75–85. https://doi.org/10.1007/s40263-016-0391-y.
Bellissima BL, Tingle MD, Cicović A, Alawami M, Kenedi C. A systematic review of clozapine-induced myocarditis. Int J Cardiol. 2018;259:122–9. https://doi.org/10.1016/j.ijcard.2017.12.102.
Anıl Yağcıoğlu AE, Ertuğrul A, Karakaşlı AA, Ağaoğlu E, Ak S, Karahan S, et al. A comparative study of detection of myocarditis induced by clozapine: with and without cardiac monitoring. Psychiatry Res. 2019;279:90–7. https://doi.org/10.1016/j.psychres.2019.07.008.
Vickers M, Ramineni V, Malacova E, Eriksson L, McMahon K, Moudgil V, et al. Risk factors for clozapine-induced myocarditis and cardiomyopathy: A systematic review and meta-analysis. Acta Psychiatr Scand. 2022;145(5):442–55. https://doi.org/10.1111/acps.13398.
Novartis Pharmaceutical Corporation. Clozaril revised package insert. 2015. https://www.novartis.com/ca-en/sites/novartis_ca/files/clozaril_scrip_e.pdf. Accessed 29 May 2023.
Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369–71. https://doi.org/10.1212/wnl.41.3.369.
Pacia SV, Devinsky O. Clozapine-related seizures: experience with 5,629 patients. Neurology. 1994;44(12):2247–9. https://doi.org/10.1212/wnl.44.12.2247.
Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457–63. https://doi.org/10.1177/070674370705200708.
Williams AM, Park SH. Seizure Associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101–11.
Nosè M, Recla E, Trifirò G, Barbui C. Antipsychotic drug exposure and risk of pneumonia: a systematic review and meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2015;24(8):812–20. https://doi.org/10.1002/pds.3804.
Dzahini O, Singh N, Taylor D, Haddad PM. Antipsychotic drug use and pneumonia: systematic review and meta-analysis. J Psychopharmacol. 2018;32(11):1167–81. https://doi.org/10.1177/0269881118795333.
Yang SY, Liao YT, Liu HC, Chen WJ, Chen CC, Kuo CJ. Antipsychotic drugs, mood stabilizers, and risk of pneumonia in bipolar disorder: a nationwide case-control study. J Clin Psychiatry. 2013;74(1):e79-86. https://doi.org/10.4088/JCP.12m07938.
Kuo C-J, Yang S-Y, Liao Y-T, Chen WJ, Lee W-C, Shau W-Y, et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophrenia Bull. 2012;39(3):648–57. https://doi.org/10.1093/schbul/sbr202.
Hung GC, Liu HC, Yang SY, Pan CH, Liao YT, Chen CC, et al. Antipsychotic reexposure and recurrent pneumonia in schizophrenia: a nested case-control study. J Clin Psychiatry. 2016;77(1):60–6. https://doi.org/10.4088/JCP.14m09301.
Chang C-K, Chen P-H, Pan C-H, Su S-S, Tsai S-Y, Chen C-C, et al. Antipsychotic medications and the progression of upper respiratory infection to pneumonia in patients with schizophrenia. Schizophrenia Res. 2020;222:327–34. https://doi.org/10.1016/j.schres.2020.05.013.
Rohde C, Siskind D, de Leon J, Nielsen J. Antipsychotic medication exposure, clozapine, and pneumonia: results from a self-controlled study. Acta Psychiatr Scand. 2020;142(2):78–86. https://doi.org/10.1111/acps.13142.
Leung JG, Hasassri ME, Barreto JN, Nelson S, Morgan RJ. Characterization of admission types in medically hospitalized patients prescribed clozapine. Psychosomatics. 2017;58(2):164–72. https://doi.org/10.1016/j.psym.2016.11.013.
Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiat. 2015;72(12):1172–81. https://doi.org/10.1001/jamapsychiatry.2015.1737.
Cicala G, Barbieri MA, Spina E, de Leon J. A comprehensive review of swallowing difficulties and dysphagia associated with antipsychotics in adults. Expert Rev Clin Pharmacol. 2019;12(3):219–34. https://doi.org/10.1080/17512433.2019.1577134.
Kulkarni DP, Kamath VD, Stewart JT. Swallowing disorders in schizophrenia. Dysphagia. 2017;32(4):467–71. https://doi.org/10.1007/s00455-017-9802-6.
Clark SR, Warren NS, Kim G, Jankowiak D, Schubert KO, Kisely S, et al. Elevated clozapine levels associated with infection: a systematic review. Schizophrenia Res. 2018;192:50–6. https://doi.org/10.1016/j.schres.2017.03.045.
Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569–74.
Wagner E, McMahon L, Falkai P, Hasan A, Siskind D. Impact of smoking behavior on clozapine blood levels—a systematic review and meta-analysis. Acta Psychiatr Scand. 2020;142(6):456–66. https://doi.org/10.1111/acps.13228.
Moschny N, Hefner G, Grohmann R, Eckermann G, Maier HB, Seifert J, et al. Therapeutic drug monitoring of second- and third-generation antipsychotic drugs-influence of smoking behavior and inflammation on pharmacokinetics. Pharmaceuticals (Basel). 2021. https://doi.org/10.3390/ph14060514.
Ronaldson KJ. Cardiovascular disease in clozapine-treated patients: evidence, mechanisms and management. CNS Drugs. 2017;31(9):777–95. https://doi.org/10.1007/s40263-017-0461-9.
Subramanian S, Völlm BA, Huband N. Clozapine dose for schizophrenia. Cochrane Database Syst Rev. 2017;6(6):Cd009555. https://doi.org/10.1002/14651858.CD009555.pub2.
Zhang D, Wang W, Li F. Association between resting heart rate and coronary artery disease, stroke, sudden death and noncardiovascular diseases: a meta-analysis. CMAJ. 2016;188(15):E384–92. https://doi.org/10.1503/cmaj.160050.
Kim DD, White RF, Barr AM, Honer WG, Procyshyn RM. Clozapine, elevated heart rate and QTc prolongation. J Psychiatry Neurosci. 2018;43(1):71–2. https://doi.org/10.1503/jpn.170135.
Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, et al. Which QT correction formulae to use for QT Monitoring? J Am Heart Assoc. 2016;5(6): e003264. https://doi.org/10.1161/JAHA.116.003264.
Nielsen J, Correll CU, Manu P, Kane JM. Termination of clozapine treatment due to medical reasons: When is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603–13. https://doi.org/10.4088/JCP.12r08064.
FDA. FDA Drug Safety Communication: FDA modifies monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. Food and Drug Administration. 2015. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-modifies-monitoring-neutropenia-associated-schizophrenia-medicine. Accessed 23 May 2023.
Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry. 2006;188:255–63. https://doi.org/10.1192/bjp.188.3.255.
Manu P, Lapitskaya Y, Shaikh A, Nielsen J. Clozapine rechallenge after major adverse effects: clinical guidelines based on 259 Cases. Am J Ther. 2018;25(2):e218–23. https://doi.org/10.1097/mjt.0000000000000715.
Mijovic A, MacCabe JH. Clozapine-induced agranulocytosis. Ann Hematol. 2020;99(11):2477–82. https://doi.org/10.1007/s00277-020-04215-y.
Silva E, Higgins M, Hammer B, Stephenson P. Clozapine rechallenge and initiation despite neutropenia—a practical, step-by-step guide. BMC Psychiatry. 2020. https://doi.org/10.1186/s12888-020-02592-2.
Giles G, Varghese S, Shymko G, Nguyen T, Waters F. Clozapine therapy and COVID-19: A systematic review of the prevalence rates, health outcomes, hematological markers, and patient perspectives. Schizophr Bull. 2023;49(1):53–67. https://doi.org/10.1093/schbul/sbac148.
Smith K, Ostinelli E, Macdonald O, Cipriani A. COVID-19 and telepsychiatry: development of evidence-based guidance for clinicians. JMIR Ment Health. 2020;7(8): e21108. https://doi.org/10.2196/21108.
Siskind D, Honer WG, Clark S, Correll CU, Hasan A, Howes O, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222–3. https://doi.org/10.1503/jpn.200061.
Verdoux H, Quiles C. Educational needs and psychoeducation interventions in clozapine users: a narrative review. Acta Psychiatr Scand. 2020;142(2):96–108. https://doi.org/10.1111/acps.13172.
Ní Dhubhlaing C, Young A, Sahm LJ. Impact of pharmacist counselling on clozapine knowledge. Schizophrenia Res Treat. 2017;2017:6120970. https://doi.org/10.1155/2017/6120970.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348: g1687. https://doi.org/10.1136/bmj.g1687.
Hynes C, Keating D, McWilliams S, Madigan K, Kinsella A, Maidment I, et al. Glasgow antipsychotic side-effects scale for clozapine —development and validation of a clozapine-specific side-effects scale. Schizophrenia Res. 2015;168(1):505–13. https://doi.org/10.1016/j.schres.2015.07.052.
Strawn JR, Keck PEJ, Stanley N, Caroff MD. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–6. https://doi.org/10.1176/ajp.2007.164.6.870.
Wells AJ, Sommi RW, Crismon ML. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm. 1988;22(6):475–80. https://doi.org/10.1177/106002808802200606.
Murch S, Tran N, Liew D, Petrakis M, Prior D, Castle D. Echocardiographic monitoring for clozapine cardiac toxicity: lessons from real-world experience. Australas Psychiatry. 2013;21(3):258–61. https://doi.org/10.1177/1039856213475684.
Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458–65. https://doi.org/10.3109/00048674.2011.572852.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for conducting this study.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Availability of Data and Material
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code availability
Not applicable.
Author Contributions
All authors made substantial contributions to the conception and design of the work. All authors worked closely together in constructing effective search strings for the different databases. Sarah Smessaert and Marc De Hert independently screened all articles and assessed their eligibility. Sarah Smessaert performed the quality assessment. Sarah Smessaert drafted the first version of the article. Marc De Hert, Johan Detraux, and Franciska Desplenter substantially revised it. All authors read and approved the final manuscript and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smessaert, S., Detraux, J., Desplenter, F. et al. Evaluating Monitoring Guidelines of Clozapine-Induced Adverse Effects: a Systematic Review. CNS Drugs 38, 105–123 (2024). https://doi.org/10.1007/s40263-023-01054-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01054-z