Abstract
Psoriasis is a common inflammatory immune disorder due to chronic activation of the adaptive and innate immune responses. Therapies for psoriasis target reducing inflammatory cytokines such as tumor necrosis factor-alpha, interleukin-17, and interleukin-22. Patients with inflammatory disorders have reduced metabolism by cytochrome P450 enzymes in the liver. The pharmacokinetic and pharmacodynamic changes due to psoriasis also have an impact on reaching therapeutic concentrations of the drug. Pharmacokinetic and pharmacodynamic data help determine the safety and clinical considerations necessary when utilizing drugs for plaque psoriasis. A literature search was performed on PubMed and Ovid MEDLINE for the pharmacokinetic and pharmacodynamic data of oral therapies and biologics utilized for moderate-to-severe plaque psoriasis. The findings from the literature search were organized into two sections: oral therapies and biologics. The pharmacokinetic and pharmacodynamic parameters in healthy patients, patients with psoriasis, and special populations are discussed in each section. The oral therapies described in this review include methotrexate, cyclosporine, apremilast, tofacitinib, and deucravacitinib. Biologics include tumor necrosis factor-alpha inhibitors, interleukin-17 inhibitors, ustekinumab, and interleukin-23 inhibitors. Clinical considerations for these therapies include drug toxicities, dosing frequency, and anti-drug antibodies. Methotrexate and cyclosporine have a risk for hepatoxicity and renal impairment, respectively. Moreover, drugs metabolized via cytochrome P450, including tofacitinib and apremilast have decreased clearance in patients with psoriasis, requiring dose adjustments. Patients treated with therapies such as adalimumab can develop anti-drug antibodies that reduce the long-term efficacy of the drug. Additionally, overweight patients benefit from more frequent dosing to achieve better psoriasis clearance.

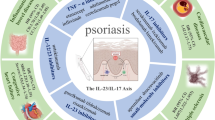
Adapted from Reis, J., Vender, R., & Torres, T. (2019). Bimekizumab: the first dual inhibitor of interleukin (IL)-17A and IL-17F for the treatment of psoriatic disease and ankylosing spondylitis. BioDrugs, 33, 391-399. Licensing number: 5595510452417
Similar content being viewed by others
References
Michalek IM, Loring B, John SM. Global report on psoriasis. Geneva: World Health Organization; 2016.
Kolli SS, Amin SD, Pona A, Cline A, Feldman SR. Psychosocial impact of psoriasis: a review for dermatology residents. Cutis. 2018;102(5S):21–5.
Stanke-Labesque F, Gautier-Veyret E, Chhun S, Guilhaumou R, French Society of Pharmacology and Therapeutics. Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther. 2020;215:107627.
Morales Castro D, Dresser L, Granton J, Fan E. Pharmacokinetic alterations associated with critical illness. Clin Pharmacokinet. 2023;62(2):209–20.
Ho VC, Griffiths CE, Berth-Jones J, Papp KA, Vanaclocha F, Dauden E, et al. Intermittent short courses of cyclosporine microemulsion for the long-term management of psoriasis: a 2-year cohort study. J Am Acad Dermatol. 2001;44(4):643–51.
Dogra S, Krishna V, Kanwar AJ. Efficacy and safety of systemic methotrexate in two fixed doses of 10 mg or 25 mg orally once weekly in adult patients with severe plaque-type psoriasis: a prospective, randomized, double-blind, dose-ranging study. Clin Exp Dermatol. 2012;37(7):729–34.
Radmanesh M, Rafiei B, Moosavi Z, Sina N. Weekly vs daily administration of oral methotrexate (MTX) for generalized plaque psoriasis: a randomized controlled clinical trial. Int J Dermatol. 2011;50(10):1291–3.
Menting SP, van Lümig PP, de Vries AQ, van den Reek JM, van der Kleij D, de Jong EM, et al. Extent and consequences of antibody formation against adalimumab in patients with psoriasis: one-year follow-up. JAMA Dermatol. 2014;150(2):130–6.
Suleiman AA, Minocha M, Khatri A, Pang Y, Othman AA. Population pharmacokinetics of risankizumab in healthy volunteers and subjects with moderate to severe plaque psoriasis: integrated analyses of phase I-III clinical trials. Clin Pharmacokinet. 2019;58:1309–21.
Menter A, Gelfand JM, Connor C, Armstrong AW, Cordoro KM, Davis DM, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–86.
Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res Ther. 2002;4(4):1–8.
Cream JJ, Pole DS. The effect of methotrexate and hydroxyurea on neutrophil chemotaxis. Br J Dermatol. 1980;102(5):557–63.
Ternowitz T, Herlin T. Neutrophil and monocyte chemotaxis in methotrexate-treated psoriasis patients. Acta Derm Venereol Suppl. 1985;120:23–6.
Weinstein GD, Velasco J. Selective action of methotrexate on psoriatic epidermal cells. J Invest Dermatol. 1972;59(1):121–7.
Jeffes EW III, McCullough JL, Pittelkow MR, McCormick A, Almanzor J, Liu G, et al. Methotrexate therapy of psoriasis: differential sensitivity of proliferating lymphoid and epithelial cells to the cytotoxic and growth-inhibitory effects of methotrexate. J Invest Dermatol. 1995;104(2):183–8.
Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard J. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322–8.
Weinstein GD, Goldfaden G, Frost P. Methotrexate: mechanism of action on DNA synthesis in psoriasis. Arch Dermatol. 1971;104(3):236–43.
Grim J, Chládek J, Martínková J. Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin Pharmacokinet. 2003;42:139–51.
Oguey D, Kölliker F, Gerber NJ, Reichen J. Effect of food on the bioavailability of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 1992;35(6):611–4.
Carneiro SC, Cassia FF, Lamy F, Chagas V, Ramos-e-Silva M. Methotrexate and liver function: a study of 13 psoriasis cases treated with different cumulative dosages. J Eur Acad Dermatol Venereol. 2008;22(1):25–9.
Saurat J, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne J, CHAMPION Study Investigators, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs methotrexate vs placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558–66.
Sbidian E, Chaimani A, Garcia-Doval I, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2022;5(5):CD011535.
Barker J, Hoffmann M, Wozel G, Ortonne J, Zheng H, Van-Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011;165(5):1109–17.
Jabbar-Lopez ZK, Yiu ZZ, Ward V, Exton LS, Mustapa MFM, Samarasekera E, et al. Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta-analysis. J Invest Dermatol. 2017;137(8):1646–54.
Rosmarin DM, Lebwohl M, Elewski BE, Gottlieb AB. Cyclosporine and psoriasis: 2008 national psoriasis foundation consensus conference. J Am Acad Dermatol. 2010;62(5):838–53.
Haider AS, Lowes MA, Suárez-Farinas M, Zaba LC, Cardinale I, Khatcherian A, et al. Identification of cellular pathways of “type 1”, Th17 T cells, and TNF-and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J Immunol. 2008;180(3):1913–20.
Faulds D, Goa KL, Benfield P. Cyclosporin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45(6):953–1040.
Maza A, Montaudié H, Sbidian E, Gallini A, Aractingi S, Aubin F, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25:19–27.
Koo J, OLP302 Study Group. A randomized, double-blind study comparing the efficacy, safety and optimal dose of two formulations of cyclosporin, Neoral and Sandimmun, in patients with severe psoriasis. Br J Dermatol. 1998;139(1):88–95.
Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque-type psoriasis: results of a multidose, double-blind trial. N Engl J Med. 1991;324(5):277–84.
Shupack J, Abel E, Bauer E, Brown M, Drake L, Freinkel R, et al. Cyclosporine as maintenance therapy in patients with severe psoriasis. J Am Acad Dermatol. 1997;36(3):423–32.
Markham T, Watson A, Rogers S. Adverse effects with long-term cyclosporin for severe psoriasis. Clin Exp Dermatol. 2002;27(2):111–4.
Schafer PH, Parton A, Capone L, Cedzik D, Brady H, Evans JF, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–29.
Schafer PH, Chen P, Fang L, Wang A, Chopra R. The pharmacodynamic impact of apremilast, an oral phosphodiesterase 4 inhibitor, on circulating levels of inflammatory biomarkers in patients with psoriatic arthritis: substudy results from a phase III, randomized, placebo-controlled trial (PALACE 1). J Immunol Res. 2015;2015:906349.
Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393–403.
Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507–17.
Cutolo M, Myerson GE, Fleischmann RM, Lioté F, Díaz-González F, Van den Bosch F, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43(9):1724–34.
Armstrong AW, Gooderham M, Warren RB, Papp KA, Strober B, Thaçi D, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39.
Azevedo A, Torres T. Tofacitinib: a new oral therapy for psoriasis. Clin Drug Invest. 2018;38:101–12.
Krishnaswami S, Boy M, Chow V, Chan G. Safety, tolerability, and pharmacokinetics of single oral doses of tofacitinib, a Janus kinase inhibitor, in healthy volunteers. Clin Pharmacol Drug Dev. 2015;4(2):83–8.
Dowty ME, Lin J, Ryder TF, Wang W, Walker GS, Vaz A, et al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab Dispos. 2014;42(4):759–73.
Ma G, Xie R, Strober B, Langley R, Ito K, Krishnaswami S, et al. Pharmacokinetic characteristics of tofacitinib in adult patients with moderate to severe chronic plaque psoriasis. Clin Pharmacol Drug Dev. 2018;7(6):587–96.
Machavaram KK, Almond LM, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, et al. A physiologically based pharmacokinetic modeling approach to predict disease–drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94(2):260–8.
Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173(4):949–61.
Papp KA, Krueger JG, Feldman SR, Langley RG, Thaci D, Torii H, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol. 2016;74(5):841–50.
Shalom G, Zisman D, Bitterman H, Harman-Boehm I, Greenberg-Dotan S, Dreiher J, et al. Systemic therapy for psoriasis and the risk of herpes zoster: a 500 000 person-year study. JAMA Dermatol. 2015;151(5):533–8.
Gadina M, Chisolm DA, Philips RL, McInness IB, Changelian PS, O’Shea JJ. Translating JAKs to jakinibs. J Immunol. 2020;204(8):2011–20.
Chimalakonda A, Burke J, Cheng L, Catlett I, Tagen M, Zhao Q, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther. 2021;11(5):1763–76.
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–62.
Catlett IM, Aras U, Hansen L, Liu Y, Bei D, Girgis IG, et al. First-in-human study of deucravacitinib: a selective, potent, allosteric small-molecule inhibitor of tyrosine kinase 2. Clin Transl Sci. 2023;16(1):151–64.
Strober B, Thaçi D, Sofen H, Kircik L, Gordon KB, Foley P, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40–51.
Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–43.
Ergun T, Seckin Gencosmanoglu D, Alpsoy E, Bulbul-Baskan E, Saricam MH, Salman A, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44(6):630–4.
Van Geel MJ, Mul K, De Jager M, Van De Kerkhof P, De Jong E, Seyger M. Systemic treatments in paediatric psoriasis: a systematic evidence-based update. J Eur Acad Dermatol Venereol. 2015;29(3):425–37.
Dogra S, Mahajan R, Narang T, Handa S. Systemic cyclosporine treatment in severe childhood psoriasis: a retrospective chart review. J Dermatol Treat. 2017;28(1):18–20.
Menter A, Cordoro KM, Davis DM, Kroshinsky D, Paller AS, Armstrong AW, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201.
Bronckers IM, Paller AS, West DP, Lara-Corrales I, Tollefson MM, Tom WL, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156(4):384–92.
Paller AS, Hong Y, Becker EM, de Lucas R, Paris M, Zhang W, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82(2):389–97.
Ruperto N, Brunner HI, Zuber Z, Tzaribachev N, Kingsbury DJ, Foeldvari I, et al. Pharmacokinetic and safety profile of tofacitinib in children with polyarticular course juvenile idiopathic arthritis: results of a phase 1, open-label, multicenter study. Pediatr Rheumatol. 2017;15(1):1–10.
AlMutairi N, Nour T. Tofacitinib in pediatric psoriasis: an open-label trial to study its safety and efficacy in children. Dermatology. 2020;236(3):191–8.
Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45(5):490–7.
Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264–72.
Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp R. Elevated tumour necrosis factor-alpha (TNF-α) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96(1):146–51.
Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34(2):161–4.
European Medicines Agency. Humira summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf. Accessed 17 Jan 2024.
Nestorov I, Zitnik R, DeVries T, Nakanishi AM, Wang A, Banfield C. Pharmacokinetics of subcutaneously administered etanercept in subjects with psoriasis. Br J Clin Pharmacol. 2006;62(4):435–45.
van der Kraaij G, Busard C, van den Reek J, Menting S, Musters A, Hutten B, et al. Adalimumab with methotrexate vs. adalimumab monotherapy in psoriasis: first-year results of a single-blind randomized controlled trial. J Invest Dermatol. 2022;142(9):2375-83.e6.
van Huizen AM, van der Kraaij GE, Busard CI, Ouwerkerk W, van den Reek JM, Menting SP, et al. Adalimumab combined with methotrexate versus adalimumab monotherapy in psoriasis: three-year follow-up data of a single-blind randomised controlled trial. J Eur Acad Dermatol Venereol. 2023;37(9):1815–24.
Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55:379–90.
Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013;133(1):17–26.
Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181(4):2799–805.
Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson AD, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523–32.
Bruin G, Hockey HP, La Stella P, Sigurgeirsson B, Fu R, Patekar M, et al. Comparison of pharmacokinetics, safety and tolerability of secukinumab administered subcutaneously using different delivery systems in healthy volunteers and in psoriasis patients. Br J Clin Pharmacol. 2020;86(2):338–51.
Reich K, Jackson K, Ball S, Garces S, Kerr L, Chua L, et al. Ixekizumab pharmacokinetics, anti-drug antibodies, and efficacy through 60 weeks of treatment of moderate to severe plaque psoriasis. J Invest Dermatol. 2018;138(10):2168–73.
Endres CJ, Salinger DH, Köck K, Gastonguay MR, Martin DA, Klekotka P, et al. Population pharmacokinetics of brodalumab in healthy adults and adults with psoriasis from single and multiple dose studies. J Clin Pharmacol. 2014;54(11):1230–8.
Bruin G, Loesche C, Nyirady J, Sander O. Population pharmacokinetic modeling of secukinumab in patients with moderate to severe psoriasis. J Clin Pharmacol. 2017;57(7):876–85.
Dragatin C, Polus F, Bodenlenz M, Calonder C, Aigner B, Tiffner KI, et al. Secukinumab distributes into dermal interstitial fluid of psoriasis patients as demonstrated by open flow microperfusion. Exp Dermatol. 2016;25(2):157–9.
Glatt S, Helmer E, Haier B, Strimenopoulou F, Price G, Vajjah P, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001.
Reich K, Blauvelt A, Armstrong A, Langley RG, de Vera A, Kolbinger F, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits low immunogenicity in psoriasis patients treated up to 5 years. J Eur Acad Dermatol Venereol. 2019;33(9):1733–41.
Bertelsen T, Ljungberg C, Litman T, Huppertz C, Hennze R, Rønholt K, et al. IκBζ is a key player in the antipsoriatic effects of secukinumab. J Allergy Clin Immunol. 2020;145(1):379–90.
Reich K, Puig L, Szepietowski JC, Paul C, Lacour JP, Tsianakas A, et al. Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study. Br J Dermatol. 2020;182(2):304–15.
Reich K, Körber A, Mrowietz U, Sticherling M, Sieder C, Früh J, et al. Secukinumab 2-weekly vs. 4-weekly dosing in patients with plaque-type psoriasis: results from the randomized GAIN study. Br J Dermatol. 2021;184(5):849–56.
Hsu S, Green LJ, Lebwohl MG, Wu JJ, Blauvelt A, Jacobson AA. Comparable efficacy and safety of brodalumab in obese and nonobese patients with psoriasis: analysis of two randomized controlled trials. Br J Dermatol. 2020;182(4):880–8.
McInnes IB, Behrens F, Mease PJ, Kavanaugh A, Ritchlin C, Nash P, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395(10235):1496–505.
Mease PJ, Smolen JS, Behrens F, Nash P, Leage SL, Li L, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–31.
Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–52.
Conti HR, Gaffen SL. IL-17–Mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. 2015;195(3):780–8.
Reddy M, Davis C, Wong J, Marsters P, Pendley C, Prabhakar U. Modulation of CLA, IL-12R, CD40L, and IL-2Rα expression and inhibition of IL-12-and IL-23-induced cytokine secretion by CNTO 1275. Cell Immunol. 2007;247(1):1–11.
Sigmundsdóttir H, Gudjonsson JE, Jónsdóttir I, Ludviksson BR, Valdimarsson H. The frequency of CLA CD8 T cells in the blood of psoriasis patients correlates closely with the severity of their disease. Clin Exp Immunol. 2001;126(2):365–9.
Wu M, Li X, Yang D, Wang M, Zhang H, Li C, et al. Comparison of pharmacokinetic similarity, immunogenicity, and safety of ustekinumab and BAT2206 in healthy Chinese male subjects in a double-blind, randomized, single-dose, parallel-group phase I Trial. BioDrugs. 2023;37(1):89–98.
Gottlieb AB, Cooper KD, McCormick TS, Toichi E, Everitt DE, Frederick B, et al. A phase 1, double-blind, placebo-controlled study evaluating single subcutaneous administrations of a human interleukin-12/23 monoclonal antibody in subjects with plaque psoriasis. Curr Med Res Opin. 2007;23(5):1081–92.
European Medicines Agency. Stelara, summary of product characteristics. 2001. https://www.ema.europa.eu/en/documents/product-information/stelara-epar-product-information_en.pdf. Accessed 17 Jan 2024.
Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–84.
Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–98.
Singh S, Kroe-Barrett RR, Canada KA, Zhu X, Sepulveda E, Wu H, et al. Selective targeting of the IL23 pathway: generation and characterization of a novel high-affinity humanized anti-IL23A antibody. MAbs. 2015;7(4):778–91.
Khatri A, Eckert D, Oberoi R, Suleiman A, Pang Y, Cheng L, et al. Pharmacokinetics of risankizumab in Asian healthy subjects and patients with moderate to severe plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis. J Clin Pharmacol. 2019;59(12):1656–68.
Yao Z, Hu C, Zhu Y, Xu Z, Randazzo B, Wasfi Y, et al. Population pharmacokinetic modeling of guselkumab, a human IgG1λ monoclonal antibody targeting IL-23, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58(5):613–27.
Yiu ZZ, Becher G, Kirby B, Laws P, Reynolds NJ, Smith CH, et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022;158(10):1131–41.
Langley RG, Tsai T, Flavin S, Song M, Randazzo B, Wasfi Y, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114–23.
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour J, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–60.
Reich K, Gooderham M, Thaçi D, Crowley JJ, Ryan C, Krueger JG, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–86.
Warren RB, Blauvelt A, Poulin Y, Beeck S, Kelly M, Wu T, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–9.
Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633–6.
Langley RG, Kasichayanula S, Trivedi M, Aras GA, Kaliyaperumal A, Yuraszeck T, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58(3):340–6.
Bodemer C, Kaszuba A, Kingo K, Tsianakas A, Morita A, Rivas E, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35(4):938–47.
Philipp S, Menter A, Nikkels AF, Barber K, Landells I, Eichenfield LF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183(4):664–72.
Jackson K, Chua L, Velez-de-Mendizabal N, Pitou C, Rodriguez Capriles C, Paller AS, et al. Population pharmacokinetic and exposure–efficacy analysis of ixekizumab in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Clin Pharmacol. 2022;88(3):1074–86.
Huang I, Yu C, Tai C, Tu Y, Chi C. Biologics for pediatric moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. JDDG. 2022;20(9):1201–9.
Bröms G, Kieler H, Ekbom A, Gissler M, Hellgren K, Lahesmaa-Korpinen A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29(3):316–27.
Chambers CD, Johnson DL, Xu R, Luo Y, Lopez-Jimenez J, Adam MP, et al. Birth outcomes in women who have taken adalimumab in pregnancy: a prospective cohort study. PLoS ONE. 2019;14(10):e0223603.
Mariette X, Förger F, Abraham B, Flynn AD, Moltó A, Flipo R, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77(2):228–33.
Clowse ME, Scheuerle AE, Chambers C, Afzali A, Kimball AB, Cush JJ, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70(9):1399–407.
Clowse ME, Förger F, Hwang C, Thorp J, Dolhain RJ, Van Tubergen A, et al. Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis. 2017;76(11):1890–6.
Hazes JM, Coulie PG, Geenen V, Vermeire S, Carbonnel F, Louis E, et al. Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use. Rheumatology. 2011;50(11):1955–68.
Baker T, Kevorkian L, Nesbitt A. FRI0162 Investigation into the binding affinity of certolizumab pegol to FcRn and functional consequences for FcRn-mediated transcytosis: comparison to infliximab, adalimumab and etanercept. Ann Rheum Dis. 2013;72(Suppl. 3):A426.
Warren RB, Reich K, Langley RG, Strober B, Gladman D, Deodhar A, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179(5):1205–7.
Egeberg A, Iversen L, Kimball AB, Kelly S, Grace E, Patel H, et al. Pregnancy outcomes in patients with psoriasis, psoriatic arthritis, or axial spondyloarthritis receiving ixekizumab. J Dermatol Treat. 2022;33(5):2503–9.
Borren NZ, Ananthakrishnan AN. Safety of biologic therapy in older patients with immune-mediated diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(9):1736-43.e4.
Megna M, Napolitano M, Balato N, Monfrecola G, Villani A, Ayala F, Balato A. Efficacy and safety of ustekinumab in a group of 22 elderly patients with psoriasis over a 2-year period. Clin Exp Dermatol. 2016;41(5):564–6.
Hayashi M, Umezawa Y, Fukuchi O, Ito T, Saeki H, Nakagawa H. Efficacy and safety of ustekinumab treatment in elderly patients with psoriasis. J Dermatol. 2014;41(11):974–80.
Phan C, Beneton N, Delaunay J, Reguiai Z, Boulard C, Fougerousse A, et al. Effectiveness and safety of anti-interleukin-17 therapies in elderly patients with psoriasis. Acta Derm Venereol. 2020;100(18):1–4.
Körber A, Papavassilis C, Bhosekar V, Reinhardt M. Efficacy and safety of secukinumab in elderly subjects with moderate to severe plaque psoriasis: a pooled analysis of phase III studies. Drugs Aging. 2018;35:135–44.
Ruggiero A, Fabbrocini G, Cinelli E, Ocampo Garza SS, Camela E, Megna M. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol. 2022;47(3):561–7.
Lee J, Pelkey R, Gubitosa J, Henrick MF, Ganz ML. Comparing healthcare costs associated with oral and subcutaneous methotrexate or biologic therapy for rheumatoid arthritis in the United States. Am Health Drug Benefits. 2017;10(1):42.
Augustin M, Reich K, Yamauchi P, Pinter A, Bagel J, Dahale S, et al. Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial. Br J Dermatol. 2022;186(6):942–54.
Gisondi P, Del Giglio M, Di Francesco V, Zamboni M, Girolomoni G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88(5):1242–7.
Lucas AT, Robinson R, Schorzman AN, Piscitelli JA, Razo JF, Zamboni WC. Pharmacologic considerations in the disposition of antibodies and antibody-drug conjugates in preclinical models and in patients. Antibodies. 2019;8(1):3.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59.
Syversen SW, Jørgensen KK, Goll GL, Brun MK, Sandanger Ø, Bjørlykke KH, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance infliximab therapy on disease control in patients with immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;326(23):2375–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of Interest/Competing Interests
Steven Feldman has received research, speaking and/or consulting support from Eli Lilly and Company, GlaxoSmithKline/Stiefel, AbbVie, Janssen, Alovtech, vTv Therapeutics, Bristol-Myers Squibb, Samsung, Pfizer, Boehringer Ingelheim, Amgen, Dermavant, Arcutis, Novartis, Novan, UCB, Helsinn, Sun Pharma, Almirall, Galderma, Leo Pharma, Mylan, Celgene, Ortho Dermatology, Menlo, Merck & Co, Qurient, Forte, Arena, Biocon, Accordant, Argenx, Sanofi, Regeneron, the National Biological Corporation, Caremark, Teladoc, BMS, Ono, Micreos, Eurofins, Informa, UpToDate, and the National Psoriasis Foundation. He is a founder and part owner of Causa Research and holds stock in Sensal Health. Jonathan Greenzaid has no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
SF and JG contributed to the conception and design of the manuscript. JG collected, analyzed, and interpreted the data and drafted the manuscript. SF critically revised the manuscript and approved the final version to be published.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greenzaid, J., Feldman, S. Clinical Pharmacokinetic and Pharmacodynamic Considerations in the Treatment of Moderate-to-Severe Psoriasis. Clin Pharmacokinet 63, 137–153 (2024). https://doi.org/10.1007/s40262-023-01341-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01341-4