Abstract
BACKGROUND:
Previous investigations have shown that local application of nanoparticles presenting the carbohydrate moiety galactose-α-1,3-galactose (α-gal epitopes) enhance wound healing by activating the complement system and recruiting pro-healing macrophages to the injury site. Our companion in vitro paper suggest α-gal epitopes can similarly recruit and polarize human microglia toward a pro-healing phenotype. In this continuation study, we investigate the in vivo implications of α-gal nanoparticle administration directly to the injured spinal cord.
METHODS:
α-Gal knock-out (KO) mice subjected to spinal cord crush were injected either with saline (control) or with α-gal nanoparticles immediately following injury. Animals were assessed longitudinally with neurobehavioral and histological endpoints.
RESULTS:
Mice injected with α-gal nanoparticles showed increased recruitment of anti-inflammatory macrophages to the injection site in conjunction with increased production of anti-inflammatory markers and a reduction in apoptosis. Further, the treated group showed increased axonal infiltration into the lesion, a reduction in reactive astrocyte populations and increased angiogenesis. These results translated into improved sensorimotor metrics versus the control group.
CONCLUSIONS:
Application of α-gal nanoparticles after spinal cord injury (SCI) induces a pro-healing inflammatory response resulting in neuroprotection, improved axonal ingrowth into the lesion and enhanced sensorimotor recovery. The data shows α-gal nanoparticles may be a promising avenue for further study in CNS trauma.
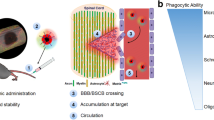
Graphical abstract
Putative mechanism of therapeutic action by α-gal nanoparticles. A. Nanoparticles injected into the injured cord bind to anti-Gal antibodies leaked from ruptured capillaries. The binding of anti-Gal to α-gal epitopes on the α-gal nanoparticles activates the complement system to release complement cleavage chemotactic peptides such as C5a, C3a that recruit macrophages and microglia. These recruited cells bind to the anti-Gal coated α-gal nanoparticles and are further polarized into the M2 state. B. Recruited M2 macrophages and microglia secrete neuroprotective and pro-healing factors to promote tissue repair, neovascularization and axonal regeneration (C.).









Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Notes
Abbreviation: SCI-spinal cord injury.
References
Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98.
Hawthorne AL, Popovich PG. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics. 2011;8:252–61.
Klebanoff SJ, Vadas MA, Harlan JM, Sparks LH, Gamble JR, Agosti JM, et al. Stimulation of neutrophils by tumor-necrosis-factor. J Immunol. 1986;136:4220–5.
Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–69.
Kong XY, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941–54.
An N, Yang J, Wang H, Sun S, Wu H, Li L, et al. Mechanism of mesenchymal stem cells in spinal cord injury repair through macrophage polarization. Cell Biosci. 2021;11:41.
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44.
Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol. 2013;133:2461–70.
Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11.
Clifford T, Finkel Z, Rodriguez B, Joseph A, Cai L. Current advancements in spinal cord injury research-glial scar formation and neural regeneration. Cells. 2023;12:853.
Hellenbrand DJ, Reichl KA, Travis BJ, Filipp ME, Khalil AS, Pulito DJ, et al. Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury. J Neuroinflammation. 2019;16:93.
Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–70.
Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–65.
Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45.
Clemente CD, Windle WF. Regeneration of severed nerve fibers in the spinal cord of the adult cat. J Comp Neurol. 1954;101:691–731.
Guth L, Zhang Z, DiProspero NA, Joubin K, Fitch MT. Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. Exp Neurol. 1994;126:76–87.
Popovich PG, Tovar CA, Wei P, Fisher L, Jakeman LB, Basso DM. A reassessment of a classic neuroprotective combination therapy for spinal cord injured rats: LPS/pregnenolone/indomethacin. Exp Neurol. 2012;233:677–85.
Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140:1–11.
Galili U, Zhu Z, Chen J, Goldufsky JW, Schaer GL. Near complete repair after myocardial infarction in adult mice by altering the inflammatory response with intramyocardial injection of alpha-gal nanoparticles. Front Cardiovasc Med. 2021;8:719160.
Kaymakcalan OE, Abadeer A, Goldufsky JW, Galili U, Karinja SJ, Dong X, et al. Topical alpha-gal nanoparticles accelerate diabetic wound healing. Exp Dermatol. 2020;29:404–13.
Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018.
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282.
Joshi M, Fehlings MG. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1 Clip design, behavioral outcomes, and histopathology. J Neurotrauma. 2002;19:175–90.
Galili U, Wigglesworth K, Abdel-Motal UM. Accelerated healing of skin burns by anti-Gal/α-gal liposomes interaction. Burns. 2010;36:239–51.
Wigglesworth KM, Racki WJ, Mishra R, Szomolanyi-Tsuda E, Greiner DL, Galili U. Rapid recruitment and activation of macrophages by anti-Gal/alpha-Gal liposome interaction accelerates wound healing. J Immunol. 2011;186:4422–32.
Galili U. Antibody production and tolerance to the alpha-gal epitope as models for understanding and preventing the immune response to incompatible ABO carbohydrate antigens and for alpha-gal therapies. Front Mol Biosci. 2023;10:1209974.
Hurwitz ZM, Ignotz R, Lalikos JF, Galili U. Accelerated porcine wound healing after treatment with α-gal nanoparticles. Plast Reconstr Surg. 2012;129:242e-e251.
Luo J, Borgens R, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. 2002;83:471–80.
Ahn M, Lee C, Jung K, Kim H, Moon C, Sim KB, et al. Immunohistochemical study of arginase-1 in the spinal cords of rats with clip compression injury. Brain Res. 2012;1445:11–9.
Gao W, Li JM. Targeted siRNA delivery reduces nitric oxide mediated cell death after spinal cord injury. J Nanobiotechnology. 2017;15:38.
Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58.
Niemi JP, DeFrancesco-Oranburg T, Cox A, Lindborg JA, Echevarria FD, McCluskey J, et al. The conditioning lesion response in dorsal root ganglion neurons is inhibited in oncomodulin knock-out mice. eNeuro. 2022;9:ENEURO-0477.
Urban MW, Ghosh B, Block CG, Strojny LR, Charsar BA, Goulao M, et al. Long-distance axon regeneration promotes recovery of diaphragmatic respiratory function after spinal cord injury. eNeuro. 2019;6:ENEURO-0096.
Carter RJ, Morton J, Dunnett SB. Motor coordination and balance in rodents. Curr Protoc Neurosci. 2001;15:8–12.
Harrison DJ, Busse M, Openshaw R, Rosser AE, Dunnett SB, Brooks SP. Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in the R6/1 Huntington’s disease mouse model. Exp Neurol. 2013;248:457–69.
Tung VW, Burton TJ, Quail SL, Mathews MA, Camp AJ. Motor performance is impaired following vestibular stimulation in ageing mice. Front Aging Neurosci. 2016;8:12.
Wagner JM, Sichler ME, Schleicher EM, Franke TN, Irwin C, Low MJ, et al. Analysis of motor function in the Tg4-42 mouse model of Alzheimer’s disease. Front Behav Neurosci. 2019;13:107.
Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284.
Ahmed RU, Alam M, Zheng YP. Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon. 2019;5:e01324.
Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52.
Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. New Engl J Med. 1990;322:1405–11.
Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the national acute spinal cord injury study. J Neurosurg. 1985;63:704–13.
Schwartz M. “Tissue-repairing” blood-derived macrophages are essential for healing of the injured spinal cord: from skin-activated macrophages to infiltrating blood-derived cells? Brain Behav Immun. 2010;24:1054–7.
Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–21.
Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198.
Shafqat A, Albalkhi I, Magableh HM, Saleh T, Alkattan K, Yaqinuddin A. Tackling the glial scar in spinal cord regeneration: new discoveries and future directions. Front Cell Neurosci. 2023;17:1180825.
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419.
Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29:3956–68.
Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–63.
Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J Neurosci. 2014;34:8904–17.
Zhang J, Li Y, Duan Z, Kang J, Chen K, Li G, et al. The effects of the M2a macrophage-induced axonal regeneration of neurons by arginase 1. Biosci Rep. 2020;40:BSR20193031.
Liu C, Li Y, Yu J, Feng L, Hou S, Liu Y, et al. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS One. 2013;8:e54841.
Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–81.
Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183:1352–63.
Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015;12:218.
Xu K, Chen QX, Li FC, Chen WS, Lin M, Wu QH. Spinal cord decompression reduces rat neural cell apoptosis secondary to spinal cord injury. J Zhejiang Univ Sci B. 2009;10:180–7.
Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–78.
Kotipatruni RR, Dasari VR, Veeravalli KK, Dinh DH, Fassett D, Rao JS. p53- and Bax-mediated apoptosis in injured rat spinal cord. Neurochem Res. 2011;36:2063–74.
Hibbs JB Jr, Vavrin Z, Taintor RR. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987;138:550–65.
Yang Z, Ming XF. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol. 2014;5:533.
Isaksson J, Farooque M, Olsson Y. Improved functional outcome after spinal cord injury in iNOS-deficient mice. Spinal Cord. 2005;43:167–70.
Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249–63.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35.
Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207.
Hackett AR, Lee JK. Understanding the NG2 glial scar after spinal cord injury. Front Neurol. 2016;7:199.
Li X, Li M, Tian L, Chen J, Liu R, Ning B. Reactive astrogliosis: implications in spinal cord injury progression and therapy. Oxid Med Cell Longev. 2020;2020:9494352.
Facchiano F, Fernandez E, Mancarella S, Maira G, Miscusi M, D’Arcangelo D, et al. Promotion of regeneration of corticospinal tract axons in rats with recombinant vascular endothelial growth factor alone and combined with adenovirus coding for this factor. J Neurosurg. 2002;97:161–8.
Dray C, Rougon G, Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci U S A. 2009;106:9459–64.
Chen W, Xia M, Guo C, Jia Z, Wang J, Li C, et al. Modified behavioural tests to detect white matter injury- induced motor deficits after intracerebral haemorrhage in mice. Sci Rep. 2019;9:16958.
Nampoothiri SS, Potluri T, Subramanian H, Krishnamurthy RG. Rodent gymnastics: neurobehavioral assays in ischemic stroke. Mol Neurobiol. 2017;54:6750–61.
Shefner JM. Strength testing in motor neuron diseases. Neurotherapeutics. 2017;14:154–60.
Grulova I, Slovinska L, Nagyova M, Cizek M, Cizkova D. The effect of hypothermia on sensory-motor function and tissue sparing after spinal cord injury. Spine J. 2013;13:1881–91.
Plemel JR, Duncan G, Chen KW, Shannon C, Park S, Sparling JS, et al. A graded forceps crush spinal cord injury model in mice. J Neurotrauma. 2008;25:350–70.
Mills CD, Grady JJ, Hulsebosch CE. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J Neurotrauma. 2001;18:1091–105.
Moore SA, Hettlich BF, Waln A. The use of an electronic von Frey device for evaluation of sensory threshold in neurologically normal dogs and those with acute spinal cord injury. Vet J. 2013;197:216–9.
Song RB, Basso DM, da Costa RC, Fisher LC, Mo X, Moore SA. von Frey anesthesiometry to assess sensory impairment after acute spinal cord injury caused by thoracolumbar intervertebral disc extrusion in dogs. Vet J. 2016;209:144–9.
Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: a role in central pain. J Neurophysiol. 2009;102:181–91.
Blomster LV, Brennan FH, Lao HW, Harle DW, Harvey AR, Ruitenberg MJ. Mobilisation of the splenic monocyte reservoir and peripheral CX(3)CR1 deficiency adversely affects recovery from spinal cord injury. Exp Neurol. 2013;247:226–40.
Acknowledgements
We thank Christa Crain (Center for Comparative Translational Research (CCTR) Senior Research Technician) for assistance during surgeries. We thank Purdue University Histology Research Laboratory (Victor Bernal-Crespo and Mackenzie J McIntosh), a core facility of the NIH-funded Indiana Clinical and Translational Science Institute for histology work. This research was partly funded by the State of Indiana and Clinical and Translational Sciences Institute (CTSI, Indiana State Department of Health (Grant # 204200 to JL) and National Institute of Neurological Disorders and Stroke R21 (No. 1R21NS115094-01). We thank Purdue Animal Behavior Core for the use of the animal behavioral equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare conflict of interest.
Ethical statement
All mice experiments including breeding, care and surgery were done in accordance to Purdue University IACUC (#1110000060).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gopalakrishnan, B., Galili, U., Saenger, M. et al. α-Gal Nanoparticles in CNS Trauma: II. Immunomodulation Following Spinal Cord Injury (SCI) Improves Functional Outcomes. Tissue Eng Regen Med 21, 437–453 (2024). https://doi.org/10.1007/s13770-023-00616-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00616-y