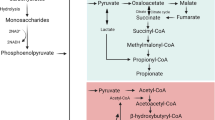
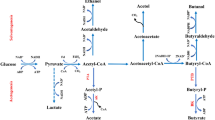
Abstract
Purpose of Review
The mechanistic understanding of the importance and the potential benefits of the gut microbiome has exploded in potential roles in human health and disease. Short chain fatty acids (SCFAs), including butyrate, are one of the key metabolic end products that has been a major focus of microbiome understanding. This brief review aims to describe butyrate’s relation to certain biological concepts and their clinical application.
Recent Findings
Butyrate has reportedly been described as a potent pro-resolution molecule that has a significant role in maintaining gut immunity, supporting gut barrier function, regulation of histone deacetylase (HDAC), and numerous systemic roles. Further research is needed to explore potential benefits of adding SCFAs for patients receiving total parenteral nutrition.
Summary
Butyrate plays several biological roles in intestinal epithelium anti-inflammatory pathways with clear benefits in numerous acute and chronic disease states and overall human health helping to maintain homeostasis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Important reference •• Very important reference
Libertucci J, Young VB. The role of the microbiota in infectious diseases. Nat Microbiol. 2019;4(1):35–45.
Rastelli M, Cani PD, Knauf C. The gut microbiome influences host endocrine functions. Endocr Rev. 2019;40(5):1271–84.
Kumar J, Rani K, Datt C. Molecular link between dietary fibre, gut microbiota and health. Mol Biol Rep. 2020;47(8):6229–37.
Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther. 2016;164:144–51.
Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;31(11):25.
Van den Abbeele P, Belzer C, Goossens M, Kleerebezem M, De Vos WM, Thas O, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7(5):949–61.
Nie K, Ma K, Luo W, Shen Z, Yang Z, Xiao M, et al. Roseburia intestinalis: a beneficial gut organism from the discoveries in genus and species. Front Cell Infect Microbiol [Internet]. 2021 [cited 2022 Sep 11];11. Available from:https://doi.org/10.3389/fcimb.2021.757718.
•• Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–26. This reference describes basic concepts of butyrate role in regulation of energy metabolism and autophagy. These basic concepts are fundamentally important in understanding the theoretical benefits and clinical applications of butyrate.
Mohamed Elfadil O, Patel J, Patel I, Ewy MW, Hurt RT, Mundi MS. Processed foods – getting back to the basics. Curr Gastroenterol Rep. 2021;23(12):20.
Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10(10):1499.
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17(12):1519–28.
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–18.
Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM. Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol Ther. 2013;139(2):189–212.
Chen J, Vitetta L. The role of butyrate in attenuating pathobiont-induced hyperinflammation. Immune Netw. 2020;20(2):e15.
Toni T, Alverdy J, Gershuni V. Re-examining chemically defined liquid diets through the lens of the microbiome. Nat Rev Gastroenterol Hepatol. 2021;18(12):903–11.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50.
Föh B, Buhre JS, Lunding HB, Moreno-Fernandez ME, König P, Sina C, et al. Microbial metabolite butyrate promotes induction of IL-10 + IgM+ plasma cells. PLoS ONE. 2022;17(3):e0266071.
Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2):432–445.e7.
• Ji J, Shu D, Zheng M, Wang J, Luo C, Wang Y, et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep. 2016;6(1):24838. This relatively recent study describes a new evidence for butyrate role in polarization of macrophages helping us to better understand the immune interplay of some disease and potential role for SCFAs.
Russell DG, Huang L, VanderVen BC. Immunometabolism at the interface between macrophages and pathogens. Nat Rev Immunol. 2019;19(5):291–304.
Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: a double-edged sword for health? Adv Nutr Bethesda Md. 2018;9(1):21–9.
Gerbeth L, Glauben R. Histone deacetylases in the inflamed intestinal epithelium—promises of new therapeutic strategies. Front Med. 2021;26(8):655956.
Evans LW, Athukorala M, Martinez-Guryn K, Ferguson BS. The role of histone acetylation and the microbiome in phytochemical efficacy for cardiovascular diseases. Int J Mol Sci. 2020;21(11):4006.
Parnham MJ, Nijkamp FP, Rossi AG. Initiation, propagation and resolution of inflammation. In: Parnham MJ, Nijkamp FP, Rossi AG, editors. Nijkamp and Parnham’s Principles of Immunopharmacology [Internet]. Cham: Springer International Publishing; 2019 [cited 2021 Oct 22]. p. 1–6. Available from: https://doi.org/10.1007/978-3-030-10811-3_1.
Chriett S, Dąbek A, Wojtala M, Vidal H, Balcerczyk A, Pirola L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep. 2019;9(1):742.
Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11(9):821–34.
Pan X, Fang X, Wang F, Li H, Niu W, Liang W, et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharmacol. 2019;176(23):4446–61.
Martin-Gallausiaux C, Larraufie P, Jarry A, Béguet-Crespel F, Marinelli L, Ledue F, et al. Butyrate produced by commensal bacteria down-regulates indolamine 2,3-dioxygenase 1 (IDO-1) expression via a dual mechanism in human intestinal epithelial cells. Front Immunol. 2018;11(9):2838.
Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–13.
Gurka MJ, Filipp SL, DeBoer MD. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutr Diabetes. 2018;8(1):1–8.
Gregory JW. Prevention of obesity and metabolic syndrome in children. Front Endocrinol. 2019;10:669.
Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 126(11):1549–64.
Müller M, Hernández MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JWE, et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep. 2019;9(1):12515.
Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, et al. Association of insulin resistance and type 2 diabetes with gut microbial diversity: a microbiome-wide analysis from population studies. JAMA Netw Open. 2021;4(7):e2118811.
McNabney SM, Henagan TM. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. 2017;9(12):E1348.
Zhang WQ, Zhao TT, Gui DK, Gao CL, Gu JL, Gan WJ, et al. Sodium butyrate improves liver glycogen metabolism in type 2 diabetes mellitus. J Agric Food Chem. 2019;67(27):7694–705.
Xiao Y, Guo Z, Li Z, Ling H, Song C. Role and mechanism of action of butyrate in atherosclerotic diseases: a review. J Appl Microbiol. 2021;131(2):543–52.
Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett. 2016;20(625):56–63.
Rode J, Yang L, König J, Hutchinson AN, Wall R, Venizelos N, et al. Butyrate rescues oxidative stress-induced transport deficits of tryptophan: potential implication in affective or gut-brain axis disorders. Neuropsychobiology. 2021;80(3):253–63.
Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, et al. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis JAD. 2020;78(2):683–97.
Stadlbauer V, Engertsberger L, Komarova I, Feldbacher N, Leber B, Pichler G, et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: a pilot study. BMC Geriatr. 2020;20(1):248.
Skonieczna-Żydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A, et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients. 2018;10(12):E1939.
Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–32.
Qiao CM, Sun MF, Jia XB, Shi Y, Zhang BP, Zhou ZL, et al. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res. 2020;387(1):111772.
Prochazkova P, Roubalova R, Dvorak J, Kreisinger J, Hill M, Tlaskalova-Hogenova H, et al. The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes. 2021;13(1):1–25.
Vieira R de S, Castoldi A, Basso PJ, Hiyane MI, Câmara NOS, Almeida RR. Butyrate attenuates lung inflammation by negatively modulating Th9 cells. Front Immunol. 2019;10:67.
Theiler A, Bärnthaler T, Platzer W, Richtig G, Peinhaupt M, Rittchen S, et al. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J Allergy Clin Immunol. 2019;144(3):764–76.
Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018;11(3):785–95.
Haak BW, Littmann ER, Chaubard JL, Pickard AJ, Fontana E, Adhi F, et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131(26):2978–86.
Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74(4):799–809.
Salvi PS, Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. 2021;10(7):1775.
Jung TH, Park JH, Jeon WM, Han KS. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract. 2015;9(4):343–9.
Frankel W, Lew J, Su B, Bain A, Klurfeld D, Einhorn E, et al. Butyrate increases colonocyte protein synthesis in ulcerative colitis. J Surg Res. 1994;57(1):210–4.
Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47(3):397–403.
Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37(4):458–66.
Rodríguez-Padilla Á, Morales-Martín G, Pérez-Quintero R, Rada-Morgades R, Gómez-Salgado J, Ruiz-Frutos C. Diversion colitis and probiotic stimulation: effects of bowel stimulation prior to ileostomy closure. Front Med. 2021;8:936.
Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320(1):23–8.
Sundaram M, Kim J. Chapter 79 - short bowel syndrome. In: Yeo CJ, editor. Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set (Eighth Edition) [Internet]. Philadelphia: Elsevier; 2019 [cited 2021 Oct 28]. p. 920–38. Available from: https://www.sciencedirect.com/science/article/pii/B9780323402323000790.
Boccia S, Torre I, Santarpia L, Iervolino C, Del Piano C, Puggina A, et al. Intestinal microbiota in adult patients with short bowel syndrome: preliminary results from a pilot study. Clin Nutr Edinb Scotl. 2017;36(6):1707–9.
Neelis E, de Koning B, Rings E, Wijnen R, Nichols B, Hulst J, et al. The gut microbiome in patients with intestinal failure: current evidence and implications for clinical practice. J Parenter Enter Nutr. 2019;43(2):194–205.
Dowhaniuk JK, Szamosi J, Chorlton S, Owens J, Mileski H, Clause RF, et al. Starving the gut: a deficit of butyrate in the intestinal ecosystem of children with intestinal failure. JPEN J Parenter Enteral Nutr. 2020;44(6):1112–23.
• Pierre JF. Gastrointestinal immune and microbiome changes during parenteral nutrition. Am J Physiol-Gastrointest Liver Physiol. 2017;312(3):G246–56. This interesting review emphasizes on the importance of understanding microbiome and GI immune function changes during PN. This understanding is critical in development of interventions that can stimulate GI immune function during PN and improve clinical outcomes.
Horwat P, Kopeć S, Garczyk A, Kaliciak I, Staręga Z, Drogowski K, et al. Influence of enteral nutrition on gut microbiota composition in patients with Crohn’s disease: a systematic review. Nutrients. 2020;12(9):2551. Ffigure.
Jirsova Z, Heczkova M, Dankova H, Malinska H, Videnska P, Vespalcova H, et al. The effect of butyrate-supplemented parenteral nutrition on intestinal defence mechanisms and the parenteral nutrition-induced shift in the gut microbiota in the rat model. BioMed Res Int. 2019;2019:7084734.
Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr. 2004;28(4):210–22; discussion 222–223.
Chen X, Xu J, Su Y, Zhu W. Effects of intravenous infusion with sodium butyrate on colonic microbiota, intestinal development- and mucosal immune-related gene expression in normal growing pigs. Front Microbiol. 2018;9:1652.
Pirozzi C, Lama A, Annunziata C, Cavaliere G, De Caro C, Citraro R, et al. Butyrate prevents valproate-induced liver injury: in vitro and in vivo evidence. FASEB J Off Publ Fed Am Soc Exp Biol. 2020;34(1):676–90.
Qiao Y li, Qian J min, Wang F rui, Ma Z yu, Wang Q wei. Butyrate protects liver against ischemia reperfusion injury by inhibiting nuclear factor kappa B activation in Kupffer cells. J Surg Res. 2014;187(2):653–9.
Zheng Y, Zhang Z, Zhang N. Protective effects of butyrate on renal ischemia-reperfusion injury in rats. Urol Int. 2019;102(3):348–55.
Zhang LT, Yao YM, Lu JQ, Yan XJ, Yu Y, Sheng ZY. Sodium butyrate prevents lethality of severe sepsis in rats: shock. 2007;27(6):672–7.
•• Wu X, Wu Y, He L, Wu L, Wang X, Liu Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J Cancer. 2018;9(14):2510–7. This review discusses an emerging evidence that butyrate has protective effect against colorectal cancer. This is critical in understanding unique roles and benefits SCFAs and for the future research on their clinical applications.
Chen J, Zhao KN, Vitetta L. Effects of Intestinal Microbial-Elaborated Butyrate on Oncogenic Signaling Pathways. Nutrients. 2019;11(5):1026.
Singh NP, Lai HC. Synergistic cytotoxicity of artemisinin and sodium butyrate on human cancer cells. Anticancer Res. 2005;25(6B):4325–31.
Dang AT, Marsland BJ. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12(4):843–50.
Gui Q, Li H, Wang A, Zhao X, Tan Z, Chen L, et al. The association between gut butyrate‐producing bacteria and non‐small‐cell lung cancer. J Clin Lab Anal [Internet]. 2020 Aug [cited 2021 Oct 20];34(8). Available from: https://doi.org/10.1002/jcla.23318.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Osman Mohamed Elfadil, Manpreet S. Mundi, Ankitaben Patel, Marwa G. Abdelmagid, Nishant Patel, and Robert Martindale have no conflicts of interest in relation to this manuscript to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohamed Elfadil, O., Mundi, M.S., Abdelmagid, M.G. et al. Butyrate: More Than a Short Chain Fatty Acid. Curr Nutr Rep 12, 255–262 (2023). https://doi.org/10.1007/s13668-023-00461-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-023-00461-4