Abstract
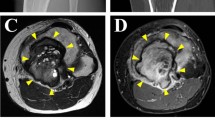
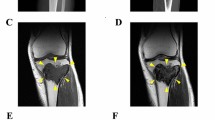
Giant cell tumor of bone (GCTB) is a rare bone tumor with osteolytic features, composed of stromal cells with a monotonous appearance, macrophages, and osteoclast-like giant cells. GCTB is commonly associated with a pathogenic mutation in the H3-3A gene. While complete surgical resection is the standard cure for GCTB, it often results in local recurrence and, rarely, metastasis. Thus, an effective multidisciplinary treatment approach is necessary. Although patient-derived cell lines is an essential tool for investigating novel treatment strategies, there are only four GCTB cell lines available in public cell banks. Therefore, this study aimed to establish novel GCTB cell lines and successfully created NCC-GCTB6-C1 and NCC-GCTB7-C1 cell lines from two patients' surgically removed tumor tissues. These cell lines exhibited H3-3A gene mutations, consistent proliferation, and invasive properties. After characterizing their behaviors, we performed high-throughput screening of 214 anti-cancer drugs for NCC-GCTB6-C1 and NCC-GCTB7-C1 and integrated their screening data with those of NCC-GCTB1-C1, NCC-GCTB2-C1, NCC-GCTB3-C1, NCC-GCTB4-C1, and NCC-GCTB5-C1 that we previously established. We identified histone deacetylase inhibitor romidepsin as a possible treatment for GCTB. These findings suggest that NCC-GCTB6-C1 and NCC-GCTB7-C1 could be valuable tools for preclinical and basic research on GCTB.






Similar content being viewed by others
References
Organisation mondiale de la sant Cidrslc (2020) Soft tissue and bone tumours
Behjati S, Tarpey PS, Presneau N, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45:1479–82.
Liede A, Bach BA, Stryker S, et al. Regional variation and challenges in estimating the incidence of giant cell tumor of bone. JBJS. 2014;96:1999.
Jamshidi K, Karimi A, Mirzaei A. Epidemiologic characteristics, clinical behavior, and outcome of the giant cell tumor of the bone: a retrospective single-center study. Arch Bone Jt Surg. 2019;7:538–44.
Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone—an overview. Arch Bone Jt Surg. 2016;4:2–9.
Borkowska AM, Szumera-Ciećkiewicz A, Szostakowski B, Pieńkowski A, Rutkowski PL. Denosumab in giant cell tumor of bone: multidisciplinary medical management based on pathophysiological mechanisms and real-world evidence. Cancers (Basel). 2022;14:2290.
Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36:1–7.
van der Heijden L, Dijkstra PDS, van de Sande MAJ, et al. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19:550–61.
Zaheer S, LeBoff M, Lewiecki EM. Denosumab for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2015;11:461–70.
Palmerini E, Chawla NS, Ferrari S, et al. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer. 2017;76:118–24.
Kato I, Furuya M, Matsuo K, Kawabata Y, Tanaka R, Ohashi K. Giant cell tumours of bone treated with denosumab: histological, immunohistochemical and H3F3A mutation analyses. Histopathology. 2018;72:914–22.
Forsyth RG, Krenács T, Athanasou N, Hogendoorn PCW. cell biology of giant cell tumour of bone: crosstalk between m/wt Nucleosome H3.3, telomeres and osteoclastogenesis. Cancers (Basel). 2021;13:5119.
Barretina J, Caponigro G, Stransky N, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7.
Garnett MJ, Edelman EJ, Heidorn SJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–5.
Folkesson E, Niederdorfer B, Nakstad VT, et al. High-throughput screening reveals higher synergistic effect of MEK inhibitor combinations in colon cancer spheroids. Sci Rep. 2020;10:11574.
Bairoch A. The cellosaurus, a cell-line knowledge resource. J Biomol Tech JBT. 2018;29:25–38.
Williams SP, McDermott U. The pursuit of therapeutic biomarkers with high-throughput cancer cell drug screens. Cell Chem Biol. 2017;24:1066–74.
Iorio F, Knijnenburg TA, Vis DJ, et al. A landscape of pharmacogenomic interactions in cancer. Cell. 2016;166:740–54.
Noguchi R, Yoshimatsu Y, Ono T, et al. Establishment and characterization of NCC-GCTB1-C1: a novel patient-derived cancer cell line of giant cell tumor of bone. Hum Cell. 2020;33:1321–8.
Yoshimatsu Y, Noguchi R, Tsuchiya R, et al. Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone. Hum Cell. 2021;34:1899–910.
Ono T, Noguchi R, Yoshimatsu Y, et al. Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone. Hum Cell. 2022;35:392–9.
Akiyama T, Yoshimatsu Y, Noguchi R, et al. Establishment and characterization of NCC-GCTB5-C1: a novel cell line of giant cell tumor of bone. Hum Cell. 2022;35:1621–9.
Billiau A, Edy VG, Heremans H, et al. Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977;12:11–5.
Beebe-Dimmer JL, Cetin K, Fryzek JP, Schuetze SM, Schwartz K. The epidemiology of malignant giant cell tumors of bone: an analysis of data from the surveillance, epidemiology and end results program (1975–2004). Rare Tumors. 2009;1: e52.
Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, George R, Matcuk J. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013;33:197–211.
Perche F, Torchilin VP. Cancer cell spheroids as a model to evaluate chemotherapy protocols. Cancer Biol Ther. 2012;13:1205–13.
Sant S, Johnston PA. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov Today Technol. 2017;23:27–36.
Grant C, Rahman F, Piekarz R, et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther. 2010;10:997–1008.
Lee HZ, Kwitkowski VE, Del Valle PL, et al. FDA approval: belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin Cancer Res. 2015;21:2666–70.
Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–52.
Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6: a018713.
West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Investig. 2014;124:30–9.
Venneker S, van Eenige R, Kruisselbrink AB, et al. Histone deacetylase inhibitors as a therapeutic strategy to eliminate neoplastic “stromal” cells from giant cell tumors of bone. Cancers (Basel). 2022;14:4708.
Yafei J, Haoran M, Wenyan J, et al. Personalized medicine modality based on patient-derived xenografts from a malignant transformed GCTB harboring H3F3A G34W mutation. J Orthop Transl. 2021;29:106–12.
Acknowledgements
We thank Drs. E. Kobayashi, S. Iwata, K. Ogura, K. Sato (Department of Musculoskeletal Oncology), Dr. Y. Takahashi (Department of Diagnostic Pathology), and the National Cancer Center Hospital for sampling tumor tissue specimens from surgically resected materials. We appreciate the technical support provided by Mrs. Y. Shiotani, Mr. N. Uchiya, and Dr. T. Imai (Central Animal Division, National Cancer Center Research Institute). We would like to thank Editage (www.editage.jp) for providing English language editing services and for their constructive comments on this manuscript. This study was technically assisted by the Fundamental Innovative Oncology Core of the National Cancer Center.
Funding
This research was supported by the Japan Agency for Medical Research and Development (Grant number: 20ck0106537h0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The ethical committee of the National Cancer Center (Tokyo, Japan) approved the use of clinical materials for this study (approval number 2004-050). Animal experiments were conducted in compliance with the guidelines of the Institute for Laboratory Animal Research, National Cancer Center Research Institute.
Informed consent
Written informed consent was provided by the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2023_928_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1 Short tandem repeat patterns of GCTB cell lines and original tumor tissues. (a) Short tandem repeat patterns of NCC-GCTB6-C1 (passage 20), (b) Short tandem repeat patterns of original tumor tissue of NCC-GCTB6-C1, (c) Short tandem repeat patterns of NCC-GCTB7-C1 (passage 20), (d) Short tandem repeat patterns of original tumor tissue of NCC-GCTB7-C1 (TIF 517 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ono, T., Noguchi, R., Yoshimatsu, Y. et al. Establishment and characterization of two novel patient-derived cell lines from giant cell tumor of bone. Human Cell 36, 1804–1812 (2023). https://doi.org/10.1007/s13577-023-00928-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00928-0