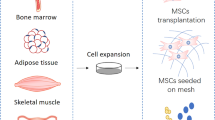
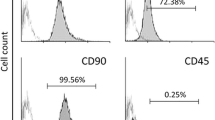
Abstract
Pelvic floor dysfunction (PFDs), which include pelvic organ prolapse (POP), stress urinary incontinence (SUI) and anal incontinence (AI), are common degenerative diseases in women that have dramatic effects on quality of life. The pathology of PFDs is based on impaired pelvic connective tissue supportive strength due to an imbalance in extracellular matrix (ECM) metabolism, the loss of a variety of cell types, such as fibroblasts, muscle cells, peripheral nerve cells, and oxidative stress and inflammation in the pelvic environment. Fortunately, exosomes, which are one of the major secretions of mesenchymal stromal cells (MSCs), are involved in intercellular communication and the modulation of molecular activities in recipient cells via their contents, which are bioactive proteins and genetic factors such as mRNAs and miRNAs. These components modify fibroblast activation and secretion, facilitate ECM modelling, and promote cell proliferation to enhance pelvic tissue regeneration. In this review, we focus on the molecular mechanisms and future directions of exosomes derived from MSCs that are of great value in the treatment of PFD.


Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- PFD:
-
Pelvic floor disorders
- POP:
-
Pelvic organ prolapse
- SUI:
-
Stress urinary incontinence
- AI:
-
Anal incontinence
- ECM:
-
Extracellular matrix
- MSC:
-
Mesenchymal stromal cell
- AI:
-
Anal incontinence
- PFMT:
-
Pelvic floor muscle physiotherapy
- BF:
-
Biofeedback
- FBR:
-
Foreign body response
- FDA:
-
Food and drug administration
- EMA:
-
European medicines agency
- HIF-1α:
-
Hypoxia-inducible factor 1α
- AGEs:
-
Advanced glycation end products
- MMPs:
-
Matrix metalloproteinases
- TIMPs:
-
Tissue inhibitors of matrix metalloproteinases
- OS:
-
Oxidative stress
- MnSOD:
-
Mitochondrial superoxide dismutase
- GPX:
-
Glutathione peroxidase
- EVs:
-
Extracellular vesicles
- TEM:
-
Transmission electron microscopy
- NTA:
-
Nanoparticle tracking analysis
- HSP70:
-
Heat shock protein 70
- TSG101:
-
Tumour-susceptibility gene 101
- MVBs:
-
Multivesicular bodies
- SNAREs:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- ESCRT:
-
The endosomal sorting complex required for transport
- MSCs-Ex:
-
Mesenchymal stromal cell-derived exosomes
- HUCMSCs:
-
Human umbilical cord mesenchymal stromal cells
- LPP:
-
Leak point pressure
- eMSCs:
-
Human endometrial mesenchymal stromal cells
- BMSCs:
-
Bone marrow mesenchymal stromal cells
- ADSC:
-
Adipose-derived stromal cell
- USC:
-
Urine-derived stromal cell (USC)
- SCs:
-
Satellite cell
- ERK:
-
Extracellular-regulated protein kinases
- SIRT1:
-
Silent mating type information regulation 2 homologue 1 (SIRT1)
- DRG:
-
Dorsal root ganglion
- SMC:
-
Smooth muscle cell
- AIF:
-
Apoptosis-inducing factor
- PARP-1:
-
Upregulating poly ADP-ribose polymerase 1
- PAR:
-
Poly ADP-ribose
- JAG1:
-
Jagged1
- ROS:
-
Reactive oxygen species
- Nrf2:
-
Nuclear factor-E2-related factor 2
- TLR4:
-
Toll-like receptor 4
- dECM:
-
Decellularized scaffold ECM
- TFF:
-
Tangential flow filtration
References
Dieter AA, Wilkins MF, Wu JM. Epidemiological trends and future care needs for pelvic floor disorders. Curr Opin Obstet Gynecol. 2015;27(5):380–4.
Kim S, Harvey MA, Johnston S. A review of the epidemiology and pathophysiology of pelvic floor dysfunction: do racial differences matter? J Obstet Gynaecol Can. 2005;27(3):251–9.
Campeau L, et al. Pelvic floor disorders: linking genetic risk factors to biochemical changes. BJU Int. 2011;108(8):1240–7.
Norton PA, et al. Genitourinary prolapse and joint hypermobility in women. Obstet Gynecol. 1995;85(2):225–8.
Cheng J, et al. Status, challenges, and future prospects of stem cell therapy in pelvic floor disorders. World J Clin Cases. 2020;8(8):1400–13.
Arnouk A, et al. Physical, complementary, and alternative medicine in the treatment of pelvic floor disorders. Curr Urol Rep. 2017;18(6):47.
Fitz FF, et al. Biofeedback for the treatment of female pelvic floor muscle dysfunction: a systematic review and meta-analysis. Int Urogynecol J. 2012;23(11):1495–516.
Alvarez J, Cvach K, Dwyer P. Complications in pelvic floor surgery. Minerva Ginecol. 2013;65(1):53–67.
Olsen AL, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6.
Sima Y, Chen Y. MSC-based therapy in female pelvic floor disorders. Cell Biosci. 2020;10:104.
Dällenbach P. To mesh or not to mesh: a review of pelvic organ reconstructive surgery. Int J Womens Health. 2015;7:331–43.
Marinaro F, et al. Meshes in a mess: Mesenchymal stem cell-based therapies for soft tissue reinforcement. Acta Biomater. 2019;85:60–74.
Shah HN, Badlani GH. Mesh complications in female pelvic floor reconstructive surgery and their management: A systematic review. Indian J Urol. 2012;28(2):129–53.
Zhang L, et al. Tension-free polypropylene mesh-related surgical repair for pelvic organ prolapse has a good anatomic success rate but a high risk of complications. Chin Med J. 2015;128(3):295–300.
Kariminekoo S, et al. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016;44(3):749–57.
Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22(3):313–25.
Li JJ, et al. Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomaterials (Basel). 2019;9(2):261.
Ribeiro-Rodrigues TM, et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res. 2017;113(11):1338–50.
Bowers DT, Brown JL. Nanofiber curvature with Rho GTPase activity increases mouse embryonic fibroblast random migration velocity. Integr Biol (Camb). 2021;13(12):295–308.
Wang LT, et al. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med. 2021;10(9):1288–303.
Ni J, et al. Therapeutic potential of human adipose-derived stem cell exosomes in stress urinary incontinence - an in vitro and in vivo study. Cell Physiol Biochem. 2018;48(4):1710–22.
Wu R, et al. Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res Ther. 2019;10(1):80.
Li Q, et al. Exosomes derived by SIRT1-overexpressing bone marrow mesenchymal stem cells improve pubococcygeus muscle injury in rats. Int J Stem Cells. 2021. https://doi.org/10.15283/ijsc21065.
Marofi F, et al. MSCs and their exosomes: a rapidly evolving approach in the context of cutaneous wounds therapy. Stem Cell Res Ther. 2021;12(1):597.
Bian D, et al. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13(1):24.
Abramowitch SD, et al. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S146–58.
Kieserman-Shmokler C, et al. From molecular to macro: the key role of the apical ligaments in uterovaginal support. Am J Obstet Gynecol. 2020;222(5):427–36.
Ruiz-Zapata AM, et al. Functional characteristics of vaginal fibroblastic cells from premenopausal women with pelvic organ prolapse. Mol Hum Reprod. 2014;20(11):1135–43.
陈义松 and 华克勤, 2008 盆底器官脱垂子宫主韧带和阴道壁的超微结构.. 上海医学 31(7):490–492.
Zhu Y, et al. Mechanical stress influences the morphology and function of human uterosacral ligament fibroblasts and activates the p38 MAPK pathway. Int Urogynecol J. 2021;33(8):2023–212.
Zhu YP, et al. Evaluation of extracellular matrix protein expression and apoptosis in the uterosacral ligaments of patients with or without pelvic organ prolapse. Int Urogynecol J. 2021;32(8):2273–81.
Ruiz-Zapata AM, et al. Extracellular matrix stiffness and composition regulate the myofibroblast differentiation of vaginal fibroblasts. Int J Mol Sci. 2020;21(13):4762.
Poncet S, et al. The expression and function of the endothelin system in contractile properties of vaginal myofibroblasts of women with uterovaginal prolapse. Am J Obstet Gynecol. 2005;192(2):426–32.
Meyer S, et al. The contractile properties of vaginal myofibroblasts: is the myofibroblasts contraction force test a valuable indication of future prolapse development? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(10):1399–403.
Zhao Y, et al. Transforming growth factor beta 1 and p44/42 expression in cardinal ligament tissues of patients with pelvic organ prolapse. Med Sci Monit. 2021;27: e930433.
Wen Y, et al. Expression of apoptotic factors in vaginal tissues from women with urogenital prolapse. Neurourol Urodyn. 2011;30(8):1627–32.
Saatli B, et al. Alteration of apoptosis-related genes in postmenopausal women with uterine prolapse. Int Urogynecol J. 2014;25(7):971–7.
Sun ZJ, et al. Proteomic analysis of the uterosacral ligament in postmenopausal women with and without pelvic organ prolapse. Chin Med J (Engl). 2015;128(23):3191–6.
Kim EJ, et al. Involvement of oxidative stress and mitochondrial apoptosis in the pathogenesis of pelvic organ prolapse. J Urol. 2013;189(2):588–94.
Chen YS, et al. Advanced glycation end products decrease collagen I levels in fibroblasts from the vaginal wall of patients with POP via the RAGE, MAPK and NF-κB pathways. Int J Mol Med. 2017;40(4):987–98.
Takacs P, et al. Uterosacral ligament smooth muscle cell apoptosis is increased in women with uterine prolapse. Reprod Sci. 2009;16(5):447–52.
Kökçü A, et al. Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet. 2002;266(2):75–8.
Cole EE, et al. Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int. 2006;97(2):345–8.
Lien KC, et al. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103(1):31–40.
Huang G, et al. Protective effect and potential mechanism of Schwann cell-derived exosomes on mechanical damage of rat dorsal root ganglion cells. J Obstet Gynaecol Res. 2021;47(10):3691–701.
Budatha M, et al. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J Clin Invest. 2011;121(5):2048–59.
Han L, et al. Association between pelvic organ prolapse and stress urinary incontinence with collagen. Exp Ther Med. 2014;7(5):1337–41.
Hu Y, et al. Expression and significance of metalloproteinase and collagen in vaginal wall tissues of patients with pelvic organ prolapse. Ann Clin Lab Sci. 2017;47(6):698–705.
Li Y, et al. Structural, functional and molecular pathogenesis of pelvic organ prolapse in patient and Loxl1 deficient mice. Aging (Albany NY). 2021;13(24):25886–902.
Jackson SR, et al. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347(9016):1658–61.
Van Doren SR. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015;44–46:224–31.
Kerkhof MH, Hendriks L, Brölmann HA. Changes in connective tissue in patients with pelvic organ prolapse–a review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):461–74.
Vetuschi A, et al. Immunolocalization of advanced glycation end products, mitogen activated protein kinases, and transforming growth factor-β/smads in pelvic organ prolapse. J Histochem Cytochem. 2018;66(9):673–86.
Li BS, et al. Role of mechanical strain-activated PI3K/Akt signaling pathway in pelvic organ prolapse. Mol Med Rep. 2016;14(1):243–53.
Dviri M, et al. Increased matrix metalloproteinases-1,-9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):113–7.
Usta A, et al. Expression of matrix metalloproteinase-1 in round ligament and uterosacral ligament tissue from women with pelvic organ prolapse. J Mol Histol. 2014;45(3):275–81.
Li L, et al. The polymorphisms of extracellular matrix-remodeling genes are associated with pelvic organ prolapse. Int Urogynecol J. 2022;33(2):267–74.
Lin T, et al. Expression of COX-2 and Nrf2/GPx3 in the anterior vaginal wall tissues of women with pelvic organ prolapse. Arch Gynecol Obstet. 2021;303(5):1245–53.
Hong S, et al. The role of GPX1 in the pathogenesis of female pelvic organ prolapse. PLoS ONE. 2017;12(8): e0181896.
Fang G, et al. Oxidative status of cardinal ligament in pelvic organ prolapse. Exp Ther Med. 2018;16(4):3293–302.
Kovács P, et al. Lithocholic acid, a metabolite of the microbiome, increases oxidative stress in breast cancer. Cancers (Basel). 2019;11(9):1255.
Liu C, et al. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse. Mol Med Rep. 2016;13(4):2999–3008.
Hong S, et al. Oxidative damage to human parametrial ligament fibroblasts induced by mechanical stress. Mol Med Rep. 2015;12(4):5342–8.
Wu K, et al. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–54.
Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29.
Drewes PG, et al. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170(2):578–89.
Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189–97.
De Broe ME, et al. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta. 1977;81(3):237–45.
Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–63.
Pan BT, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–8.
Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844–51.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
Camussi G, et al. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1(1):98–110.
Livshits MA, et al. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319.
Zhang H, et al. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:1464.
Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871–81.
Chang YH, et al. Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transpl. 2018;27(3):349–63.
Kowal EJK, et al. Extracellular vesicle isolation and analysis by western blotting. Methods Mol Biol. 2017;1660:143–52.
Jung MK, Mun JY. Sample preparation and imaging of exosomes by transmission electron microscopy. J Vis Exp. 2018. https://doi.org/10.3791/56482.
Dragovic RA, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7(6):780–8.
Su T, et al. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance. ACS Nano. 2019;13(2):2450–62.
Moghadasi S, et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):302.
Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7(7):495–504.
Heijnen HF, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–9.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208.
Jadli AS, et al. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467(1–2):77–94.
Munich S, et al. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1(7):1074–83.
Tian T, et al. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–95.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641. https://doi.org/10.3402/jev.v3.24641.
Zhou Y, et al. Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscip Rev RNA. 2016;7(6):758–71.
van den Boorn JG, et al. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29(4):325–6.
Mao M, et al. Human umbilical cord mesenchymal stem cells reconstruct the vaginal wall of ovariectomized Sprague-Dawley rats: implications for pelvic floor reconstruction. Cell Tissue Res. 2021;386(3):571–83.
Ma Y, et al. Mesenchymal stem cell-based bioengineered constructs enhance vaginal repair in ovariectomized rhesus monkeys. Biomaterials. 2021;275: 120863.
Edwards SL, et al. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater. 2015;13:286–94.
Kinebuchi Y, et al. Autologous bone-marrow-derived mesenchymal stem cell transplantation into injured rat urethral sphincter. Int J Urol. 2010;17(4):359–68.
Corcos J, et al. Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn. 2011;30(3):447–55.
Huang X, et al. Core-shell poly(l-lactic acid)-Hyaluronic acid nanofibers for cell culture and pelvic ligament tissue engineering. J Biomed Nanotechnol. 2021;17(3):399–406.
Jin M, et al. Transplantation of bone marrow-derived mesenchymal stem cells expressing elastin alleviates pelvic floor dysfunction. Stem Cell Res Ther. 2016;7(1):51.
Jin M, et al. MicroRNA-29 facilitates transplantation of bone marrow-derived mesenchymal stem cells to alleviate pelvic floor dysfunction by repressing elastin. Stem Cell Res Ther. 2016;7(1):167.
Wu X, et al. Acceleration of pelvic tissue generation by overexpression of basic fibroblast growth factor in stem cells. Connect Tissue Res. 2022;63(3):256–68.
Zhuang G, et al. Secretomes of human pluripotent stem cell-derived smooth muscle cell progenitors upregulate extracellular matrix metabolism in the lower urinary tract and vagina. Stem Cell Res Ther. 2021;12(1):228.
Zhou M, et al. M2 Macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int Immunopharmacol. 2021;101(Pt B): 108223.
Liu X, et al. Exosomes secreted by adipose-derived mesenchymal stem cells regulate type I collagen metabolism in fibroblasts from women with stress urinary incontinence. Stem Cell Res Ther. 2018;9(1):159.
Kisby CK, et al. Impact of repeat dosing and mesh exposure chronicity on exosome-induced vaginal tissue regeneration in a porcine mesh exposure model. Female Pelvic Med Reconstr Surg. 2021;27(3):195–201.
Zhou M, et al. Effects of RSC96 schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit. 2018;24:7841–9.
Zhang J, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
Zhang B, et al. HucMSC-exosome mediated-wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68.
Kisby CK, et al. Exosome-induced vaginal tissue regeneration in a porcine mesh exposure model. Female Pelvic Med Reconstr Surg. 2021;27(10):609–15.
Xing H, et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnology. 2021;19(1):264.
Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15(4):193–203.
Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49(1):59–68.
Sugimura-Wakayama Y, et al. Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells Dev. 2015;24(22):2687–99.
Haupt M, et al. Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl Med. 2021;10(3):357–73.
Jun EK, et al. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci. 2014;15(1):605–28.
Eirin A, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64.
Hu Y, et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021;19(1):150.
Kim S, et al. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119.
Shi Y, et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther. 2015;6(1):120.
Wang X, et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int. 2019;2019:2402916.
Zhao G, et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res Ther. 2020;11(1):174.
Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int J Mol Med. 2006;17(4):681–5.
Monroe MN, et al. Extracellular vesicles influence the pulmonary arterial extracellular matrix in congenital diaphragmatic hernia. Pediatr Pulmonol. 2020;55(9):2402–11.
Lee YI, et al. Randomized controlled study for the anti-aging effect of human adipocyte-derived mesenchymal stem cell media combined with niacinamide after laser therapy. J Cosmet Dermatol. 2021;20(6):1774–81.
Cabral J, et al. Extracellular vesicles as modulators of wound healing. Adv Drug Deliv Rev. 2018;129:394–406.
Kwon TR, et al. Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol Photoimmunol Photomed. 2016;32(3):120–8.
Chamberlain CS, et al. Exosome-educated macrophages and exosomes differentially improve ligament healing. Stem Cells. 2021;39(1):55–61.
Xu J, et al. Infrapatellar fat pad mesenchymal stromal cell-derived exosomes accelerate tendon-bone healing and intra-articular graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2022;50(3):662–73.
Guo Z, et al. Exosomal MATN3 of urine-derived stem cells ameliorates intervertebral disc degeneration by antisenescence effects and promotes NPC proliferation and ECM synthesis by activating TGF-β. Oxid Med Cell Longev. 2021;2021:5542241.
Zhang B, et al. HucMSC exosome-delivered 14-3-3ζ orchestrates self-control of the wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34(10):2485–500.
De Gregorio C, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11(1):168.
Villatoro AJ, et al. Canine colostrum exosomes: characterization and influence on the canine mesenchymal stem cell secretory profile and fibroblast anti-oxidative capacity. BMC Vet Res. 2020;16(1):417.
Li X, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14.
Dalirfardouei R, et al. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–68.
Sung SE, et al. Mesenchymal stem cell exosomes derived from feline adipose tissue enhance the effects of anti-inflammation compared to fibroblasts-derived exosomes. Vet Sci. 2021;8(9):182.
Liu W, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):259.
da Silva Meirelles L, et al. Improving the therapeutic ability of mesenchymal stem/stromal cells for the treatment of conditions influenced by immune cells. Stem Cells Int. 2019;2019:6820395.
Li P, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804.
Tian Y, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9(1):1697028.
Théry C, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006. https://doi.org/10.1002/0471143030.cb0322s30.
Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22(3):1428–42.
Jiang L, et al. Exosomes derived from TSG-6 modified mesenchymal stromal cells attenuate scar formation during wound healing. Biochimie. 2020;177:40–9.
Ding J, et al. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019;2019:9742765.
Li B, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814–26.
Khayambashi P, et al. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci. 2021;22(2):684. https://doi.org/10.3390/ijms22020684.
Chen P, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–59.
Kim YG, Choi J, Kim K. Mesenchymal stem cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnol J. 2020;15(12): e2000082.
Qazi TH, et al. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials. 2017;140:103–14.
Shi Q, et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904.
Yang J, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed. 2020;15:5911–26.
Xiong Y, et al. All-in-one: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18(1): e2104229.
Kim HY, et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243: 119942.
Kim JY, et al. Defined MSC exosome with high yield and purity to improve regenerative activity. J Tissue Eng. 2021;12:20417314211008624.
Wang L, et al. Preparation of engineered extracellular vesicles derived from human umbilical cord mesenchymal stem cells with ultrasonication for skin rejuvenation. ACS Omega. 2019;4(27):22638–45.
Joo HS, et al. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727. https://doi.org/10.3390/ijms21030727.
Cao J, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11(1):206.
Haraszti RA, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26(12):2838–47.
Wu Z, He D, Li H. Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact Mater. 2021;6(3):823–35.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and Technolog Commission of Shanghai Municipality (No.21Y11906700 to Yising Chen). Science Commission of Shangha and Technology Municipality (No.20Y11907300 to Yisong Chen).
Author information
Authors and Affiliations
Contributions
LMX drafted the manuscript; CZX contributed to the analysis of the results; and YZSM and YSC reviewed and modified the manuscript. All authors agreed on the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Sima, Y., Xiao, C. et al. Exosomes derived from mesenchymal stromal cells: a promising treatment for pelvic floor dysfunction. Human Cell 36, 937–949 (2023). https://doi.org/10.1007/s13577-023-00887-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00887-6