Abstract
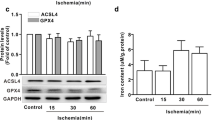
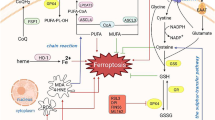
The SLC7A11/GPX4 axis plays an important role in ferroptosis during cardiac ischemia/reperfusion injury (IRI). The present study was designed to evaluate the impact of dexmedetomidine (DEX) post-conditioning on cardiac IRI and to explore whether the effect was achieved by SLC7A11/GPX4 signaling pathway regulation. Rat myocardial IRI was established by occluding the left anterior descending artery for 30 min followed by 2-h reperfusion. The infarct area was detected by diphenyltetrazolium chloride (TTC) staining; the cardiac function was evaluated by echocardiography. The levels of lipid peroxide biomarkers were measured to estimate the injury caused by lipid peroxide. HE staining and Sirius staining were utilized to assess myocardial damage and fibrosis. The mitochondrial morphology was observed by electron micrography. Western blot and quantitative real-time polymerase chain reaction were employed to measure the relative molecular characteristics. Our results showed that DEX administration at the beginning of reperfusion attenuated IRI-induced myocardial injury, alleviated mitochondrial dysfunction, decreased the level of reactive oxygen species (ROS), alleviated mitochondrial dysfunction, inhibited the activation of SLC7A11/GPX4, and modulated the expression of ferroptosis-related proteins, including SLC7A11, glutathione peroxidase 4 (GPX4), ferritin heavy chain (FTH), and cyclooxygenase-2 (COX-2). Conversely, the ferroptosis activator erastin partly suppressed the DEX-mediated cardio protection. Altogether, these results reveal that DEX inhibits ferroptosis by enhancing the expression of SLC7A11 and GPX4, thereby preventing cardiac I/R injury.







Similar content being viewed by others
Data availability statement
Research data are not shared.
References
Landoni G, Bignami E, Oliviero F, Zangrillo A. Halogenated anaesthetics and cardiac protection in cardiac and non-cardiac anaesthesia. Ann Card Anaesth. 2009;12:4–9.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Stockwell BR, FriedmannAngeli JP, Bayir H, Bush AI, Conrad M, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Liu MR, Zhu WT, Pei DS. System Xc-: a key regulatory target of ferroptosis in cancer. Investig New Drugs. 2021;39:1123–31.
Song X, Zhu S, Chen P, Hou W, Wen Q, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc-activity. Curr Biol CB. 2018;28:2388-2399.e5.
Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–70.
Gong Y, Wang N, Liu N, Dong H. Lipid peroxidation and gpx4 inhibition are common causes for myofibroblast differentiation and ferroptosis. DNA Cell Biol. 2019;38:725–33.
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308.
Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosisin cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314:H659–68.
Fang X, Wang H, Han D, Xie E, Yang X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–80.
Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs. 2015;75:1119–30.
Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32:1322–6.
Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42.
Kong W, Kang K, Gao Y, Liu H, Meng X, et al. Dexmedetomidine alleviates LPS-induced septic cardiomyopathy via the cholinergic anti-inflammatory pathway in mice. Am J Transl Res. 2017;9:5040–7.
Wang Y, Wu S, Yu X, Zhou S, Ge M, et al. Dexmedetomidine protects rat liver against ischemia-reperfusion injury partly by the α2A-adrenoceptor subtype and the mechanism is associated with the TLR4/NF-κB pathway. Int J Mol Sci. 2016;17:995.
Chang JH, Jin MM, Liu JT. Dexmedetomidine pretreatment protects the heart against apoptosis in ischemia/reperfusion injury in diabetic rats by activating PI3K/Akt signaling in vivo and in vitro. Biomed Pharmacother Biomed Pharmacother. 2020;127:110188.
Wang C, Yuan W, Hu A, Lin J, Xia Z, et al. Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22:175–84.
Alakoski T, Ulvila J, Yrjölä R, Vainio L, Magga J, et al. Inhibition of cardiomyocyte Sprouty1 protects from cardiac ischemia-reperfusion injury. Basic Res Cardiol. 2019;114:7.
Peng K, Chen WR, Xia F, Liu H, Meng XW, et al. Dexmedetomidine post-treatment attenuates cardiac ischaemia/reperfusion injury by inhibiting apoptosis through HIF-1α signalling. J Cell Mol Med. 2020;24:850–61.
Zhong Y, Li YP, Yin YQ, Hu BL, Gao H. Dexmedetomidine inhibits pyroptosis by down-regulating miR-29b in myocardial ischemia reperfusion injury in rats. Int Immunopharmacol. 2020;86:106768.
Wu ZL, Davis J, Zhu Y. Dexmedetomidine protects against myocardial ischemia/reperfusion injury by ameliorating oxidative stress and cell apoptosis through the Trx1-dependent Akt pathway. BioMed Res Int. 2020. https://doi.org/10.1155/2020/8979270.
Li W, Li W, Leng Y, Xiong Y, Xia Z, et al. is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39:210–25.
Arcangeli A, D’Alò C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2019;10:687–95.
Xue BB, Chen BH, Tang YN, Weng CW, Lin LN. Dexmedetomidine protects against lung injury induced by limb ischemia-reperfusion via the TLR4/MyD88/NF-κB pathway. Kaohsiung J Med Sci. 2019;35:672–8.
Ibacache M, Sanchez G, Pedrozo Z, Galvez F, Humeres C, et al. Dexmedetomidine preconditioning activates pro-survival kinases and attenuates regional ischemia/reperfusion injury in rat heart. Biochim Biophys Acta. 2012;1822:537–45.
Yoshitomi O, Cho S, Hara T, Shibata I, Maekawa T, et al. Direct protective effects of dexmedetomidine against myocardial ischemia-reperfusion injury in anesthetized pigs. Shock (Augusta, GA). 2012;38:92–7.
Mahboobi SK. Dexmedetomidine and renal protection after cardiac surgery. J Clin Anesth. 2020;40:121–2.
Qiu L, Ge L, Hu Q. dexmedetomidine protects SK-N-SH nerve cells from oxidative injury by maintaining iron homeostasis. Biol Pharm Bull. 2020;43:424–31.
Hao S, Yu J, He W, Huang Q, Zhao Y, et al. cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia (New York, NY). 2017;19:1022–32.
Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J Off Publ Feder Am Soc Exp Biol. 2005;19:1088–95.
Xie Y, Hou W, Song X, Yu Y, Huang J, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79.
Li N, Wang W, Zhou H, Wu Q, Duan M, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radical Biol Med. 2020;160:303–18.
Gao M, Jiang X. To eat or not to eat-the metabolic flavor of ferroptosis. Curr Opin Cell Biol. 2018;51:58–64.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
Gao M, Yi J, Zhu J, Minikes AM, Monian P, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354-363.e3.
He Y, Yang Z, Li J, Li E. Dexmedetomidine reduces the inflammation and apoptosis of doxorubicin-induced myocardial cells. Exp Mol Pathol. 2020;113:104371.
He L, Wang Z, Zhou R, Xiong W, Yang Y, et al. Dexmedetomidine exerts cardioprotective effect through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the NF-κB pathway. Biomed Pharmacother Biomed Pharmacother. 2021;133:110993.
Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas E, et al. Antioxidants and human diseases. Clin Chim Acta Int J Clin Chem. 2014;436:332–47.
Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2020. https://doi.org/10.1080/15548627.2020.1810918.
Dong H, Qiang Z, Chai D, Peng J, Xia Y, et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging. 2020;12:12943–59.
Fang X, Cai Z, Wang H, Han D, Cheng Q, et al. Loss of cardiac ferritin h facilitates cardiomyopathy via slc7a11-mediated ferroptosis. Circ Res. 2020;127:486–501.
Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x(c)(-): cystine supplier and beyond. Amino Acids. 2012;42:231–46.
Liu T, Jiang L, Tavana O, Gu W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Can Res. 2019;79:1913–24.
Guan X, Li Z, Zhu S, Cheng M, Ju Y, et al. Galangin attenuated cerebral ischemia-reperfusion injury by inhibition of ferroptosis through activating the SLC7A11/GPX4 axis in gerbils. Life Sci. 2021;264:118660.
Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys. 2020;680:108241.
Sung HK, Song E, Jahng J, Pantopoulos K, Sweeney G. Iron induces insulin resistance in cardiomyocytes via regulation of oxidative stress. Sci Rep. 2019;9:4668.
Acknowledgements
This study was supported by grants from Jiangsu Province department of science and technology (BE2020634, BK20191138 to J.Z), Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (BJ2020049 to J.Z), the Natural Science Foundation in Jiangxi Province grant (20192ACBL21037 to P.Y.), the National Natural Science Foundation of China (81760048 to J.Z. and 81760050 to P.Y.)
Author information
Authors and Affiliations
Contributions
PY, JZ, JRZ, YD, DDC, HJS, FLY, SYL, XZL, PPL, LHF, and SCY performed nearly all the experiments. PY, JZ, YD, and DDC performed hemodynamic study and analyzed the data. PY, JZ, and JRZ designed the whole study. PY, SYL, and XZL were the major contributors in writing the manuscript, and all the experiments were performed under their guidance. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, P., Zhang, J., Ding, Y. et al. Dexmedetomidine post-conditioning alleviates myocardial ischemia–reperfusion injury in rats by ferroptosis inhibition via SLC7A11/GPX4 axis activation. Human Cell 35, 836–848 (2022). https://doi.org/10.1007/s13577-022-00682-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00682-9