Abstract
Background
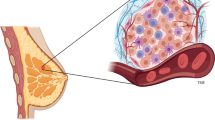
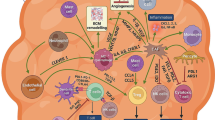
Breast cancer (BC), the second most common cause of cancer-related deaths, remains a significant threat to the health and wellness of women worldwide. The tumor microenvironment (TME), comprising cellular components, such as cancer-associated fibroblasts (CAFs), immune cells, endothelial cells and adipocytes, and noncellular components such as extracellular matrix (ECM), has been recognized as a critical contributor to the development and progression of BC. The interplay between TME components and cancer cells promotes phenotypic heterogeneity, cell plasticity and cancer cell stemness that impart tumor dormancy, enhanced invasion and metastasis, and the development of therapeutic resistance. While most previous studies have focused on targeting cancer cells with a dismal prognosis, novel therapies targeting stromal components are currently being evaluated in preclinical and clinical studies, and are already showing improved efficacies. As such, they may offer better means to eliminate the disease effectively.
Conclusions
In this review, we focus on the evolving concept of the TME as a key player regulating tumor growth, metastasis, stemness, and the development of therapeutic resistance. Despite significant advances over the last decade, several clinical trials focusing on the TME have failed to demonstrate promising effectiveness in cancer patients. To expedite clinical efficacy of TME-directed therapies, a deeper understanding of the TME is of utmost importance. Secondly, the efficacy of TME-directed therapies when used alone or in combination with chemo- or radiotherapy, and the tumor stage needs to be studied. Likewise, identifying molecular signatures and biomarkers indicating the type of TME will help in determining precise TME-directed therapies.





Similar content being viewed by others
References
L. Rebecca, Siegel, Kimberly D. Miller, Ahmedin Jemal, Cancer Statistics, 2019, CA Cancer J Clin 69, 7–34 (2019)
H. Sung, J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J Clin 71, 209–249 (2021)
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours, Nature 490, 61-70 (2012)
H.-P. Sinn and H. Kreipe, A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition, Breast Care 8, 149–154 (2013). https://doi.org/10.1159/000350774
O. Gluz, Liedtke C, Gottschalk N, Triple-negative breast cancer—current status and future directions. Ann Oncol 20, 1913–1927 (2009)
M.B. Allinen, RameenCai, Li Brennan, Cameron Lahti-Domenici, Jaana Huang, Haiyan Porter, Dale Hu, Min Chin, Lynda Richardson, Andrea, Molecular characterization of the tumor microenvironment in breast cancer, Cancer Cell 6, 17-32 (2004)
H. Dvorak, Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing, N Engl J Med 315, 1650–1659 (1986)
Y. Xiong, McDonald LT, Russell DL, Kelly RR, Wilson KR, Mehrotra M, Soloff and L.A. AC, Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment, World J Stem Cells 7, 253–265 (2015)
C.C. Maley, A. Aktipis, T.A. Graham, A. Sottoriva, A.M. Boddy, M. Janiszewska, A.S. Silva, M. Gerlinger, Y. Yuan, K.J. Pienta, K.S. Anderson, R. Gatenby, C. Swanton, D. Posada, C.I. Wu, J.D. Schiffman, E.S. Hwang, K. Polyak, A.R.A. Anderson, J.S. Brown, M. Greaves, D. Shibata, Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer 17, 605–619 (2017). https://doi.org/10.1038/nrc.2017.69
K.G.K. Deepak, R. Vempati, G.P. Nagaraju, V.R. Dasari, S. Nagini, D.N. Rao and R.R. Malla, Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer, Pharmacol Res 153, 104683 (2020)
K.E. Mao Y, Garfield DH, Shen K, Wang J, Stromal cells in tumour microenvironment and breast cancer, Cancer Metast Rev 32, 303–315 (2013)
L. Vannucci, Stroma as an active player in the development of the tumor microenvironment, Cancer Microenv 8, 159–166 (2015)
H.N. Armel, Kamdje, Jean Marc Muller, Kiven Erique Lukong Signaling pathways in breast cancer: Therapeutic targeting of the microenvironment, Cell Signal 26, 2843-2856 (2014)
C.F. Singer, D. Gschwantler-Kaulich, A. Fink-Retter, C. Haas, G. Hudelist, K. Czerwenka, E. Kubista, Differential gene expression profile in breast cancer-derived stromal fibroblasts. Breast Cancer Res Treat 110, 273–281 (2008)
R. Eftekhari, R. Esmaeili, R. Mirzaei, K. Bidad, S. de Lima, M. Ajami, H. Shirzad, J. Hadjati, K. Majidzadeh-A, Study of the tumor microenvironment during breast cancer progression. Cancer Cell Int 17, 123 (2017)
T. Li, J. Fu, Z. Zeng, D. Cohen, J. Li, Q. Chen, B. Li and X.S. Liu, TIMER2. 0 for analysis of tumor-infiltrating immune cells, Nucl Acids Res 48, W509-W514 (2020)
J.M. Houthuijzen, J. Jonkers, Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metast Rev 37, 577–597 (2018)
A. Liotta L, Kohn EC, The microenvironment of the tumour-host interface, Nature 411, 375–379 (2001)
C.E. Weber, Kothari, A. N.,Wai, P. Y., Li, N. Y., Driver, J., Zapf, M. A., Osteopontin mediates an MZF1-TGF-beta1- dependent transformation of mesenchymal stem cells into cancerassociated fibroblasts in breast cancer, Oncogene 34, 4821–4833 (2015)
A. Avgustinova, Iravani M, Robertson D, Fearns A, Gao Q, Klingbeil P, Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun 7, 10305 (2016)
R. Lappano, D.C. Rigiracciolo, A. Belfiore, M. Maggiolini, E.M. De Francesco, Cancer associated fibroblasts: Role in breast cancer and potential as therapeutic targets. Expert Opin Ther Targets 24, 559–572 (2020)
P.S. Soon, E. Kim, C.K. Pon, A.J. Gill, K. Moore, A.J. Spillane, D.E. Benn, R.C. Baxter, Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr Relat Cancer 20, 1–12 (2013)
K. Kessenbrock, V. Plaks, Z. Werb, Matrix metalloproteinases: regulators ofthe tumor microenvironment. Cell 141, 52–67 (2010)
C. Jedeszko, B.C. Victor, I. Podgorski, B.F. Sloane, Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ. Cancer Res 69, 9148–9155 (2009)
S.-W. Tyan, W.-H. Kuo, C.-K. Huang, C.-C. Pan, J.-Y. Shew, K.-J. Chang, Y.H.P.L. Eva and W.-H. Lee, Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis, PloS One 6, e15313 (2011)
Y.P. Choi, J.H. Lee, M.Q. Gao, B.G. Kim, S. Kang, S.H. Kim, N.H. Cho, Cancer-associated fibroblast promote transmigration through endothelial brain cells in three-dimensional in vitro models. Int J Cancer 135, 2024–2033 (2014)
G. Hu, P. Cheng, J. Pan, S. Wang, Q. Ding, Z. Jiang, L. Cheng, X. Shao, L. Huang, J. Huang, An IL6–adenosine positive feedback loop between CD73+ γδTregs and CAFs promotes tumor progression in human breast cancer. Cancer Immunol Res 8, 1273 (2020). https://doi.org/10.1158/2326-6066.CIR-19-0923
G. Hu, P. Cheng, J. Pan, S. Wang, Q. Ding, Z. Jiang, L. Cheng, X. Shao, L. Huang and J. Huang, An IL6–adenosine positive feedback loop between CD73<sup>+</sup> γδTregs and CAFs promotes tumor progression in human breast cancer, Cancer Immunol Res 8, 1273–1286 (2020). https://doi.org/10.1158/2326-6066.CIR-19-0923
P.H. Chang, Hwang-Verslues W.W, Chang Y. C, Chen C. C, Hsiao M, Jeng Y M, et al., Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway, Cancer Res 72, 4652–4661 (2012)
Y. Chen, Zhang X, Pivotal regulators of tissue homeostasis and cancer: macrophages, Exp Hematol Oncol 6, 23 (2017)
A. Sica, A. Mantovani, Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122, 787–795 (2012)
E.R. Matteo Santonia, Tiziana Saladinoa, et al, Triple negative breast cancer: Key role of tumor-associated macrophages in regulating the activity of anti-PD-1/PD-L1 agents, BBA - Rev Cancer 1869, 78–84 (2018)
C. Yang, L. He, P. He, Y. Liu, W. Wang, Y. He, Y. Du, F. Gao, Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol 32, 14 (2015)
A. Mantovani, F. Marchesi, A. Malesci, L. Laghi, P. Allavena, Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14, 399–416 (2017)
U. Mehraj, H. Qayoom and M.A. Mir, Prognostic significance and targeting tumor-associated macrophages in cancer: new insights and future perspectives, Breast Cancer 28, 539-555 (2021)
H. Li, B. Yang, J. Huang, Y. Lin, T. Xiang, J. Wan, H. Li, S. Chouaib, G. Ren, Cyclooxygenase-2 in tumor-associated macrophages promotes breast cancer cell survival by triggering a positive-feedback loop between macrophages and cancer cells. Oncotarget 6, 29637 (2015)
L. Gan, Z. Qiu, J. Huang, Y. Li, H. Huang, T. Xiang, J. Wan, T. Hui, Y. Lin, H. Li, Cyclooxygenase-2 in tumor-associated macrophages promotes metastatic potential of breast cancer cells through Akt pathway. Int J Biol Sci 12, 1533 (2016)
J. Joyce, Pollard JW. , Microenvironmental regulation of metastasis, Nat Rev Cancer 9, 239–252 (2009)
S. Sangaletti, Di Carlo E, Gariboldi S, Miotti S, Cappetti B, Parenza M, et al., Macrophage-derived SPARC bridges tumour cell-extracellular matrix interactions toward metastasis, Cancer Res 68, 9050–9059 (2008)
T. Kitamura, Qian B-Z, Soong D, Cassetta L, Noy R, Sugano G, et al., CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212, 1043–1059 (2012)
B. Qian, Li J, Zhang H, Kitamura T, Zhang J, Liam R, et al., CCL2 recruits inflammatory monocytes to facilitate breast tumour metastasis. Nature 475, 222–225 (2011)
Y. Togashi, K. Shitara, H. Nishikawa, Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol 16, 356–371 (2019)
K. Wing, Sakaguchi S, Regulatory T cells exert checks and balances on self-tolerance and autoimmunity. Nat Immunol 11, 7–13 (2010)
S.E.A. Onizuka, Tumor rejection by in vivo administration of anti- CD25 (interleukin-2 receptor alpha) monoclonal antibody, Cancer Res 59, 3128–3133 (1999)
J. Shimizu, Yamazaki S, Sakaguchi S, Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 163, 5211–5218 (1999)
S. Sakaguchi, Miyara M, Costantino C. M, Hafler D A, FOXP3+ regulatory T cells in the human immune system, Nat Rev Immunol 10, 490–500 (2010)
Y.N.H. Togashi, Regulatory T cells: molecular and cellular basis for immunoregulation, Curr Top Microbiol Immunol 410, 3–27 (2017)
M. Gobert, Treilleux, I., Bendriss-Vermare, N. et al, Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome, Cancer Res 69, 2000 (2009)
L.W.E.A. Collison, The inhibitory cytokine IL-35 contributes to regulatory T cell function, Nature 450, 566–569 (2007)
M.E.E.A. Turnis, Interleukin-35 limits anti- tumor immunity, Immunity 44, 316–329 (2016)
W. Tan, Zhang W, Strasner A, et al., Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature 470, 548–553 (2011)
N.M. Clark, L.M. Martinez, S. Murdock, J.T. deLigio, A.L. Olex, C. Effi, M.G. Dozmorov and P.D. Bos, Regulatory T cells support breast cancer progression by opposing IFN-γ-Dependent functional reprogramming of myeloid cells, Cell Rep 33, 108482 (2020)
Y. Xu, X. Dong, P. Qi, Y. Ye, W. Shen, L. Leng, L. Wang, X. Li, X. Luo, Y. Chen, Sox2 communicates with Tregs through CCL1 to promote the stemness property of breast cancer cells. Stem Cells 35, 2351–2365 (2017)
C. Ni, Q.-Q. Fang, W.-Z. Chen, J.-X. Jiang, Z. Jiang, J. Ye, T. Zhang, L. Yang, F.-B. Meng, W.-J. Xia, Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+ γδ1 Treg cells. Signal Transduct Target Ther 5, 1–14 (2020)
A. Mantovani, M. A. Cassatella, C. Costantini, and S. Jaillon, Neutrophils in the activation and regulation of innate and adaptive immunity, Nature Rev Immunol 11, 519–531 (2011)
Y. Tsuda, H. Takahashi, M. Kobayashi, T. Hanafusa, D.N. Herndon, F. Suzuki, Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21, 215–226 (2004)
H.A.K. Kolarova, S. Kremserova et al., Myeloperoxidase induces the priming of platelets. Free Radical Biol Med 61, 357–369 (2013)
F. Donskov, Immunomonitoring and prognostic relevance of neutrophils in clinical trials, Semin Cancer Biol 23, 200–207 (2013)
I. Mishalian, R. Bayuh, L. Levy, L. Zolotarov, J. Michaeli, Z.G. Fridlender, Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother 62, 1745–1756 (2013)
Z.G. Fridlender, J. Sun, I. Mishalian, S. Singhal, G. Cheng, V. Kapoor, W. Horng, G. Fridlender, R. Bayuh, G.S. Worthen, S.M. Albelda, Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One 7, e31524 (2012). https://doi.org/10.1371/journal.pone.0031524
J. Jablonska, S. Leschner, K. Westphal, S. Lienenklaus, S. Weiss, Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest 120, 1151–1164 (2010)
H.T. Snoderly, B.A. Boone, M.F. Bennewitz, Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res 21, 1–13 (2019)
M. Demers, D.S. Krause, D. Schatzberg, K. Martinod, J.R. Voorhees, T.A. Fuchs, D.T. Scadden, D.D. Wagner, Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA 109, 13076–13081 (2012)
J. Park , Wysocki RW, Amoozgar Z, et al., Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps, Sci Transl Med 8, 361 (2016)
A. Teijeira, S. Garasa, M.C. Ochoa, M. Villalba, I. Olivera, A. Cirella, I. Eguren-Santamaria, P. Berraondo, K.A. Schalper, C.E. de Andrea, IL8, neutrophils, and NETs in a collusion against cancer immunity and immunotherapy. Clin Cancer Res. 27, 2383–2393 (2021)
S.K. Wculek, I. Malanchi, Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015)
P.F. Yu, Y. Huang, Y.Y. Han, L.Y. Lin, W.H. Sun, A.B. Rabson, Y. Wang, Y.F. Shi, TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene 36, 482–490 (2017)
M.P. Shekhar et al., Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res 61, 1320–1326 (2001)
P. Carmeliet, Jain R. K., Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000)
D. Hanahan, Weinberg R. A., Hallmarks of cancer, the next generation. Cell 144, 646–674 (2001)
S. Mittal, N.J. Brown, I. Holen, The breast tumor microenvironment: role in cancer development, progression and response to therapy. Expert Rev Mol Diagn 18, 227–243 (2018)
P. Ghiabi, J. Jiang, J. Pasquier, M. Maleki, N. Abu-Kaoud, N. Halabi, B.S. Guerrouahen, S. Rafii, A. Rafii, Breast cancer cells promote a notch-dependent mesenchymal phenotype in endothelial cells participating to a pro-tumoral niche. J Transl Med 13, 1–19 (2015)
J. Zarrer, M.-T. Haider, D.J. Smit, H. Taipaleenmäki, Pathological crosstalk between metastatic breast cancer cells and the bone microenvironment. Biomolecules 10, 337 (2020)
E.M. Zeisberg, S. Potenta, L. Xie, M. Zeisberg, R. Kalluri, Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67, 10123–10128 (2007)
S. Acharyya, Oskarsson T, Vanharanta S, et al, A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 (2011)
A. Yadav, Kumar B, Yu J-G, Old M, Teknos TN, Kumar P. , Tumor-associated endothelial cells promote tumor metastasis by chaperoning circulating tumor cells and protecting them from anoikis, PLoS One 10, (2015)
S. Toor , Elkord E, Myeloid-derived suppressor cells, eLS:1–8 (2015)
P. Sinha, Clements VK, Ostrand-Rosenberg S, Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol 174, 636–645 (2005)
S. Kusmartsev, Li Y, Chen SH, Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation, J Immunol 165, 779–785 (200)
E. Suzuki, Kapoor V, Jassar AS, Kaiser LR, Albelda SM, Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11, 6713–6721 (2005)
K.D. Simpson, D.J. Templeton and J.V. Cross, Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment, J Immunol 189, 5533–5540 (2012). https://doi.org/10.4049/jimmunol.1201161
A.M.K. Law, Lim E, Ormandy C.J, Gallego-Ortega D, The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy, Endocr Relat Cancer 24, R123–R144 (2017)
J. Yu , Wang Y, Yan F, Zhang P et.al, Noncanonical NF-κB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer, J Immunol 193, 2574–2586 (2014)
J. Waight, Netherby C , Hensen ML, Miller A, et.al, Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis, J Clin Invest 10, 4464–4478 (2013)
J.T. Erler, K.L. Bennewith, T.R. Cox, G. Lang, D. Bird, A. Koong, Q.T. Le, A.J. Giaccia, Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009). https://doi.org/10.1016/j.ccr.2008.11.012
K. Oh, O.Y. Lee, S.Y. Shon, O. Nam, P.M. Ryu, M.W. Seo, D.S. Lee, A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res 15, R79 (2013). https://doi.org/10.1186/bcr3473
D. Peng, T. Tanikawa, W. Li, L. Zhao, L. Vatan, W. Szeliga, S. Wan, S. Wei, Y. Wang, Y. Liu, Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res 76, 3156–3165 (2016)
Y. Wang, Lehuédé C, Laurent V, et al., Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer, Cancer Lett 324, 142–151 (2012)
J. Tan, Buache E, Chenard MP, et al., Adipocyte is a non-trivial, dynamic partner of breast cancer cells, Int J Dev Biol 55, 851–859 (2011)
K. Andarawewa, Motrescu ER, Chenard MP, et al., Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front, Cancer Res 65, 10862–11087 (2005)
B. Dirat, Bochet L, Dabek M, et al., Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion, Cancer Res 71, 2455–2465 (2011)
I. Kimijima, Ohtake T, Sagara H, et al. , Scattered fat invasion: an indicator for poor prognosis in premenopausal, and for positive estrogen receptor in postmenopausal breast cancer patients, Oncology 55, 25–30 (2000)
J. Yamaguchi, Ohtani H, Nakamura K, et al., Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast, Am J Clin Pathol 130, 382–388 (2008)
L. Lapeire, Hendrix A, Lambein K, et al., Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling, Cancer Res 74, 6806–6819 (2014)
P. Iyengar, Espina V, Williams TW, et al., Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment, J Clin Invest 115, 1163–1176 (2005)
L. Vona-Davis, Rose DP, Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14, 189–206 (2007)
L. Bochet et al., Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun 411, 102–106 (2011)
J. Ligibel, Obesity and breast cancer, Oncology 5, 994–1000 (2011)
B. Dirat et al., Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev 19, 45–52 (2010)
D. Roy, Walsh LA: Candidate prognostic markers in breast cancer: focus on extracellular proteases and their inhibitors. Breast Cancer 6, 81–91 (2014)
T. Oskarsson, Extracellular matrix components in breast cancer progression and metastasis, Breast 22, 66–72 (2013)
P. Lu, Weaver VM, Werb Z, The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196, 395–406 (2012)
M. Yamauchi, M. Terajima and M. Shiiba, in Post-Translational Modification of Proteins, (Springer, 2019), p. 309–324
M.K. Jena and J. Janjanam, Role of extracellular matrix in breast cancer development: a brief update, F1000Res 7, 274 (2018). https://doi.org/10.12688/f1000research.14133.2
Y.H. Levental KR, Kass L, Lakins JN, et al., Matrix crosslinking forces tumor progression by enhancing integrin signaling, Cell 139, 891–906 (2009)
P. Schedin, Keely PJ. , Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. , Cold Spring Harb. Perspect. Biol 3, a00322 (2011)
I. Acerbi, Cassereau L, Dean I, Shi Q, Au A, Park C, et al., Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration, Integr Biol 7, 1120–1134 (2015)
M. García-Mendoza, Inman D, Ponik SM, et al., Neutrophils drive accelerated tumor progression in the collagen-dense mammary tumor microenvironment, Breast Cancer Res 18, 4 (2016)
A. Sieminski, Hebbel RP, Gooch KJ, The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res 297, 574–584 (2004)
J. Kohn, Zhou DW, Bordeleau F, et al., Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior, Biophys J 108, 471–478 (2015)
T. Cox, Rumney RM, Schoof EM, et al., The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase, Nature 522, 106–110 (2015)
M. Hayashi, Yamamoto, Y, Ibusuki, M, et al., Evaluation of tumor stiffness by elastography is predictive for pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer, Ann Surg Oncol 19, 3042–3049 (2012)
M. Giussani, G. Merlino, V. Cappelletti, E. Tagliabue, M.G. Daidone, Tumor-extracellular matrix interactions: Identification of tools associated with breast cancer progression. Semin Cancer Biol 35, 3–10 (2015). https://doi.org/10.1016/j.semcancer.2015.09.012
J. Insua-Rodríguez, Oskarsson Thordur, The extracellular matrix in breast cancer. Adv Drug Deliv Rev 97, 41–55 (2016)
S. Tiainen, A. Masarwah, S. Oikari, K. Rilla, K. Hämäläinen, M. Sudah, A. Sutela, R. Vanninen, J. Ikonen, R. Tammi, Tumor microenvironment and breast cancer survival: combined effects of breast fat, M2 macrophages and hyaluronan create a dismal prognosis. Breast Cancer Res Treat 179, 565–575 (2020)
B.P. Toole, Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4, 528–539 (2004)
Z. Wang, Y. Wu, H. Wang, Y. Zhang, L. Mei, X. Fang, X. Zhang, F. Zhang, H. Chen, Y. Liu, Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA 111, E89–E98 (2014)
L. Chen, L.Y.W. Bourguignon, Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer 13, 1–13 (2014)
P. Auvinen, K. Rilla, R. Tumelius, M. Tammi, R. Sironen, Y. Soini, V.-M. Kosma, A. Mannermaa, J. Viikari, R. Tammi, Hyaluronan synthases (HAS1–3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat 143, 277–286 (2014)
H. Koyama, T. Hibi, Z. Isogai, M. Yoneda, M. Fujimori, J. Amano, M. Kawakubo, R. Kannagi, K. Kimata and S.i. Taniguchi, Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M, Am J Pathol 170, 1086-1099 (2007)
S. Libring, A. Shinde, M.K. Chanda, M. Nuru, H. George, A.M. Saleh, A. Abdullah, T.L. Kinzer-Ursem, S. Calve, M.K. Wendt, The dynamic relationship of breast cancer cells and fibroblasts in fibronectin accumulation at primary and metastatic tumor sites. Cancers 12, 1270 (2020)
J. Nolan, A.F. Mahdi, C.P. Dunne and P.A. Kiely, Collagen and fibronectin promote an aggressive cancer phenotype in breast cancer cells but drive autonomous gene expression patterns, Gene 761, 145024 (2020)
P. Qian, Z. Zuo, Z. Wu, X. Meng, G. Li, Z. Wu, W. Zhang, S. Tan, V. Pandey, Y. Yao, Pivotal role of reduced let-7g expression in breast cancer invasion and metastasis. Cancer Res 71, 6463–6474 (2011)
S. Wu, Q. Zheng, X. Xing, Y. Dong, Y. Wang, Y. You, R. Chen, C. Hu, J. Chen, D. Gao, Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. J Exp Clin Cancer Res 37, 1–12 (2018)
A. Holle, Young JL, Spatz JP, In vitro cancer cell-ECM interactions inform in vivo cancer treatment. Adv Drug Deliv Rev 97, 270–279 (2016)
H. Al, Muhammad Wicha, Max S Benito-Hernandez, Adalberto Morrison, Sean J Clarke, Michael F, Prospective identification of tumorigenic breast cancer cells, Proc Natl Acad Sci USA 100, 3983-3988 (2003)
Y.M. Kinugasa, Takahiro Takakura, Nobuyuki, CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells 32, 145–156 (2014)
U. Mehraj, A.H. Dar, N.A. Wani and M.A. Mir, Tumor microenvironment promotes breast cancer chemoresistance, Cancer Chemother Pharmacol 87, 147-158 (2021)
X.L. Zhao, Y. Lin, J. Jiang, Z. Tang, S. Yang, L. Lu et al., Highmobilitygroup box 1 released by autophagic cancer-associatedfibroblasts maintains the stemness of luminal breast cancer cells. J Pathol 243, 376–389 (2017)
K. Amornsupak, T. Insawang, P. Thuwajit, P. O-Charoenrat, S.A. Eccles, C.Thuwajit,, Cancer-associated fibroblasts induce high mobility group box 1 andcontribute to resistance to doxorubicin in breast cancer cells, BMC Cancer 14, 955 (2014)
K.L. Mueller, J.M. Madden, G.L. Zoratti, C. Kuperwasser, K. List, J.L. Boerner, Fibroblast-secreted hepatocyte growth factor mediates epidermal growth factor receptor tyrosine kinase inhibitor resistance in triple-negative breast cancersthrough paracrine activation of Met Breast Cancer Res 14, 1 (2012)
X. Sun, Y. Mao, J. Wang, L. Zu, M. Hao, G. Cheng et al., IL-6 secreted bycancer-associated fibroblasts induces tamoxifen resistance in luminal breast cancer. Oncogene 33, 4450 (2014)
M. Loeffler, J.A. Kruger, A.G. Niethammer, R.A. Reisfeld, Targeting tumorassociatedfibroblasts improves cancer chemotherapy by increasingintratumoral drug uptake. J Clin Invest 116, 1955–1962 (2006)
D.J. Waugh, Wilson, Catherine The interleukin-8 pathway in cancer. Clin Cancer Res 14, 6735–6741 (2008)
J. Scheller, Rose-John Stefan Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol 195, 173–183 (2006)
D.H.H.A. Iliopoulos, Struhl Kevin An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139, 693–706 (2009)
H. Korkaya, Liu Suling, Wicha Max S, Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 121, 3804–3809 (2011)
W. Chen, Y. Qin, S. Liu, Cytokines, breast cancer stem cells (BCSCs) and chemoresistance. Clin Transl Med 7, 27 (2018)
J. Li, K. He, P. Liu, L.X. Xu, Iron participated in breast cancer chemoresistance by reinforcing IL-6 paracrine loop. Biochem Biophys Res Commun 475, 154–160 (2016)
K. Wang, X. Zhu, K. Zhang, Y. Yin, Y. Chen and T. Zhang, Interleukin‐6 contributes to chemoresistance in MDA‐MB‐231 cells via targeting HIF‐1α, J Biochem Mol Toxicol 32, e22039 (2018)
P.-C. Wang, C.-C. Weng, Y.-S. Hou, S.-F. Jian, K.-T. Fang, M.-F. Hou, K.-H. Cheng, Activation of VCAM-1 and its associated molecule CD44 leads to increased malignant potential of breast cancer cells. Int J Mol Sci 15, 3560–3579 (2014)
S. Saha, S. Mukherjee, P. Khan, K. Kajal, M. Mazumdar, A. Manna, S. Mukherjee, S. De, D. Jana, D.K. Sarkar, Aspirin suppresses the acquisition of chemoresistance in breast cancer by disrupting an NFκB–IL6 signaling axis responsible for the generation of cancer stem cells. Cancer Res 76, 2000–2012 (2016)
Q. Si-Qi, B, Stijn J.H. Waaijera, Mieke C. Zwagerc, Elisabeth G.E. de Vriesa, Bert van der Vegtc, Carolien P. Schrödera. , Tumour-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat Rev 70, 178–189 (2018)
O. Olson, Kim H, Quail DF, Foley EA, Joyce JA., Tumour-associated macrophages suppress the cytotoxic activity of antimitotic agents, Cell Rep 19, 101–113 (2017)
L. Ireland, A. Santos, F. Campbell, C. Figueiredo, D. Hammond, L.G. Ellies, U. Weyer-Czernilofsky, T. Bogenrieder, M. Schmid, A. Mielgo, Blockade of insulin-like growth factors increases efficacy of paclitaxel in metastatic breast cancer. Oncogene 37, 2022–2036 (2018)
T. Chanmee, Ontong Pawared, Kimata Koji, Itano Naoki Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol 5, 180 (2015)
G.L. Semenza, The hypoxic tumor microenvironment: A driving force for breast cancer progression, Mol Cell Res 1863, 382–391 (2016)
C.C. Wong, Gilkes Daniele M, Zhang Huafeng, et al., Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA 108, 16369–16374 (2011)
H. Akbulut, Babahan Cansu, Abgarmi Samira Abdi, et al., Recent advances in cancer stem cell targeted therapy, Crit Rev Oncogen 24, (2019)
F. Kong, Gao F, Li H, Liu H, Zhang Y, CD47: a potential immunotherapy target for eliminating cancer cells. Clin Transl Oncol 18, 1051–1055 (2016)
P. Zhu, F. He, Y. Hou, G. Tu, Q. Li, T. Jin, H. Zeng, Y. Qin, X. Wan and Y. Qiao, A novel hypoxic long noncoding RNA KB-1980E6. 3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-Myc mRNA stability, Oncogene 40, 1609–1627 (2021)
A. Tajbakhsh, Hasanzadeh M, Rezaee M, Khedri M, Khazaei M, et al., Therapeutic potential of novel formulated forms of curcumin in the treatment of breast cancer by the targeting of cellular and physiological dysregulated pathways, J Cell Physiol 233, 2183-2192 (2017)
K. Velaei, Samadi N, Barazvan B, Soleimani Rad J., Tumour microenvironment- mediated chemoresistance in breast cancer, Breast 30, 92–100 (2016)
Witkiewicz, AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, and S.M. et al., An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol 174, 2023–2034 (2009)
S. Togo, Polanska UM, Horimoto Y, Orimo A, Carcinoma-associated fibroblasts are a promising therapeutic target, Cancers 5, 149-169 (2013)
M. Salamone, Pavia FC, Ghersi G, Proteolytic enzymes clustered in specialized plasma-membrane domains drive endothelial cells’ migration, PloS One 11, e0154709 (2016)
E.E.A. Ostermann, Effective immunoconjugate therapy in cancer models targeting a serine protease of tumour fibroblasts, Clin Cancer Res 14, 4584–4592 (2008)
A.M. LeBeau, Brennen W. N, Aggarwal S, Denmeade S. R, Targeting the cancer stroma with a fibroblast activation protein- activated promelittin protoxin, Mol Cancer Ther 8, 1378–1386 (2009)
I.E.A. Mercier, Implications for the response to hormonal therapy, Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumour suppressor functional inactivation. Cancer Biol Ther 7, 1212–1225 (2008)
A. Dittmer et al., Mesenchymal stem cells and carcinoma-associated fibroblasts sensitize breast cancer cells in 3D cultures to kinase inhibitors., Int J Oncol 39, 689–696 (2011)
S. Tang, Y. Hou, H. Zhang, G. Tu, L. Yang, Y. Sun, L. Lang, X. Tang, Y.-E. Du, M. Zhou, Oxidized ATM promotes abnormal proliferation of breast CAFs through maintaining intracellular redox homeostasis and activating the PI3K-AKT. MEK-ERK, and Wnt-β-catenin signaling pathways, Cell Cycle 14, 1908–1924 (2015)
O. Aprelikova, Palla J, Hibler B, Yu X, Greer Y, Yi M et al., Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumour cell motility. Oncogene 32, 3246–3253 (2013)
C. Verbaanderd, H. Maes, M.B. Schaaf, V.P. Sukhatme, P. Pantziarka, V. Sukhatme, P. Agostinis and G. Bouche, Repurposing drugs in oncology (ReDO)—chloroquine and hydroxychloroquine as anti-cancer agents, Cancer Med Sci 11, (2017)
Jotzu, C., Alt, E.,Welte, G., Li, J., Hennessy, B. T., Devarajan, E,, Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors., Cell Oncol 34, 55–67 (2011)
R. Harris, Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung, Inflammopharmacol 17, 55–67 (2009)
C. Capparelli, Chiavarina B, Whitaker-Menezes D et al., CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts,“fueling” tumour growth via paracrine interactions, without an increase in neo-angiogenesis., Cell Cycle 11, 3599–3610 (2012)
S. Su, J. Chen, H. Yao, J. Liu, S. Yu, L. Lao, M. Wang, M. Luo, Y. Xing, F. Chen, CD10+ GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856 (2018)
T. Hagemann, Lawrence T, McNeish I, et al., “Re-educating” tumour-associated macrophages by targeting NF-κB. J Exp Med 205, 1261–1268 (2008)
R. Mukhtar, Nseyo O, Campbell MJ, Esserman LJ, Tumour-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev Mol Diagn 11, 91–100 (2011)
M.A. Mir, U. Mehraj, Double-crosser of the immune system: Macrophages in tumor Ppogression and metastasis. Curr Immunol Rev 15, 172–184 (2019)
X. Tang, Tumour-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer, Cancer Lett 332, 3–10 (2013)
K.Y. Lu, X Chemokine (CC motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone, J Biol Chem 284, 29087–29096 (2009)
M.W. Usman, J. Gao, T. Zheng, C. Rui, T. Li, X. Bian, H. Cheng, P. Liu, F. Luo, Macrophages confer resistance to PI3K inhibitor GDC-0941 in breast cancer through the activation of NF-κB signaling. Cell Death Dis 9, 1–12 (2018)
P. Sharma, Allison JP, The future of immune checkpoint therapy. Science 348(6230):56–61. Sica A, Mantovani A. 2012. Macrophage plasticity and polarization: in vivo veritas, J Clin Invest 122, 787–795 (2015)
R. Vonderheide, LoRusso PM, Khalil M, et al., Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulatory expression on patient T cells, Clin Cancer Res 16, 3485–3494 (2010)
K.P. Papadopoulos, L. Gluck, L.P. Martin, A.J. Olszanski, A.W. Tolcher, G. Ngarmchamnanrith, E. Rasmussen, B.M. Amore, D. Nagorsen, J.S. Hill, J. Stephenson Jr., First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody. Patients with advanced solid tumors, Clin Cancer Res 23, 5703–5710 (2017). https://doi.org/10.1158/1078-0432.Ccr-16-3261
M.A. Cannarile, M. Weisser, W. Jacob, A.M. Jegg, C.H. Ries, D. Ruttinger, Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 5, 53 (2017). https://doi.org/10.1186/s40425-017-0257-y
M. Tariq, J. Zhang, G. Liang, L. Ding, Q. He, B. Yang, Macrophage polarization: Anti-cancer strategies to target tumor-associated macrophages in breast cancer. J Cell Biochem 118, 2484–2501 (2017). https://doi.org/10.1002/jcb.25895
G. Deep, R. Agarwal, Targeting tumor microenvironment with silibinin: promise and potential for a translational cancer chemopreventive strategy. Curr Cancer Drug Targets 13, 486–499 (2013). https://doi.org/10.2174/15680096113139990041
G. Genard, S. Lucas, C. Michiels, Reprogramming of tumor-associated macrophages with anticancer therapies: Radiotherapy versus chemo- and immunotherapies. Front Immunol 8, 828 (2017). https://doi.org/10.3389/fimmu.2017.00828
D.R. Bhatia, S. Rath and S. Gupta, in Unravelling Cancer Signaling Pathways: A Multidisciplinary Approach, (Springer, 2019), p. 539–583
J. Yang, J. Yan, B. Liu, Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol 9, 978 (2018). https://doi.org/10.3389/fimmu.2018.00978
T. Iwai, Y. Harada, H. Saeki, E. Oki, Y. Maehara, Y. Yonemitsu, Capecitabine reverses tumor escape from anti-VEGF through the eliminating CD11bhigh/Gr1high myeloid cells. Oncotarget 9, 17620 (2018)
E. Rietkotter, K. Menck, A. Bleckmann, K. Farhat, M. Schaffrinski, M. Schulz, U.K. Hanisch, C. Binder, T. Pukrop, Zoledronic acid inhibits macrophage/microglia-assisted breast cancer cell invasion. Oncotarget 4, 1449–1460 (2013). https://doi.org/10.18632/oncotarget.1201
R. Yuan, S. Li, H. Geng, X. Wang, Q. Guan, X. Li, C. Ren, X. Yuan, Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int Immunopharmacol 49, 30–37 (2017). https://doi.org/10.1016/j.intimp.2017.05.014
N. Xue, Q. Zhou, M. Ji, J. Jin, F. Lai, J. Chen, M. Zhang, J. Jia, H. Yang, J. Zhang, W. Li, J. Jiang, X. Chen, Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci Rep 7, 39011 (2017). https://doi.org/10.1038/srep39011
R. Deng, S.M. Wang, T. Yin, T.H. Ye, G.B. Shen, L. Li, J.Y. Zhao, Y.X. Sang, X.G. Duan, Y.Q. Wei, Dimethyl sulfoxide suppresses mouse 4T1 breast cancer growth by modulating tumor-associated macrophage differentiation. J Breast Cancer 17, 25–32 (2014). https://doi.org/10.4048/jbc.2014.17.1.25
M. Genin, F. Clement, A. Fattaccioli, M. Raes, C. Michiels, M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15, 577 (2015). https://doi.org/10.1186/s12885-015-1546-9
A. Ribas, Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol 37, 450–454 (2010). https://doi.org/10.1053/j.seminoncol.2010.09.010
K. Sugamura, N. Ishii, A.D. Weinberg, Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol 4, 420–431 (2004). https://doi.org/10.1038/nri1371
S.L. Buchan, A. Rogel, A. Al-Shamkhani, The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 131, 39–48 (2018). https://doi.org/10.1182/blood-2017-07-741025
Y. Li, F. Li, F. Jiang, X. Lv, R. Zhang, A. Lu and G. Zhang, A mini-review for cancer immunotherapy: Molecular understanding of PD-1/PD-L1 pathway & translational blockade of immune checkpoints, Int J Mol Sci 17, (2016). https://doi.org/10.3390/ijms17071151
M.M. Zhan, X.Q. Hu, X.X. Liu, B.F. Ruan, J. Xu, C. Liao, From monoclonal antibodies to small molecules: the development of inhibitors targeting the PD-1/PD-L1 pathway. Drug Discov Today 21, 1027–1036 (2016). https://doi.org/10.1016/j.drudis.2016.04.011
S.J. Luen, P. Savas, S.B. Fox, R. Salgado, S. Loi, Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 49, 141–155 (2017). https://doi.org/10.1016/j.pathol.2016.10.010
Y. Chang, Y. Jiang, Y. Chen, X. Xing, Y. Zhou, T. Sang, J. Li, A. Zhao, J. Zhang, J. Zhao, Y. Liu, C. Zheng, Bone marrow PD-1 positive T cells reflect tumor mass and prognosis in multiple myeloma. Int J Clin Exp Pathol 11, 304–313 (2018)
S. Sau, A. Petrovici, H.O. Alsaab, K. Bhise and A.K. Iyer, PDL-1 antibody drug conjugate for selective chemo-guided immune modulation of cancer, Cancers 11, (2019). https://doi.org/10.3390/cancers11020232
A. Polk, I.M. Svane, M. Andersson, D. Nielsen, Checkpoint inhibitors in breast cancer - Current status. Cancer Treat Rev 63, 122–134 (2018). https://doi.org/10.1016/j.ctrv.2017.12.008
L. Wein, S.J. Luen, P. Savas, R. Salgado, S. Loi, Checkpoint blockade in the treatment of breast cancer: current status and future directions. Br J Cancer 119, 4–11 (2018). https://doi.org/10.1038/s41416-018-0126-6
P. D’Arrigo, M. Tufano, A. Rea, V. Vigorito, N. Novizio, S. Russo, M.F. Romano, S. Romano, Manipulation of the immune system for cancer defeat: a focus on the T cell inhibitory checkpoint molecules. Curr Med Chem (2018). https://doi.org/10.2174/0929867325666181106114421
R. Nanda, L.Q. Chow, E.C. Dees, R. Berger, S. Gupta, R. Geva, L. Pusztai, M. Dolled-Filhart, K. Emancipator and E.J. Gonzalez, p. 9–13
B. Curti, Kovacsovics-Bankowski M, Morris N, et al., OX40 is a potent immune-stimulating target in latestage cancer patients. Cancer Res 73, 7189–7198 (2013)
S. Burugu, Gao D, Leung S, Chia SK, Nielsen TO, LAG- 3+ tumour infiltrating lymphocytes in breast cancer: clinical correlates and association with PD-1/PD-L1+ tumours, Ann Oncol 28, 2977–2984 (2017)
K. Sakuishi, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC, Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumour immunity. J Exp Med 10, 2187–2194 (2010)
M. Dougan, Dougan S, Slisz J, et al., IAP inhibitors enhance co-stimulation to promote tumor immunity, J Exp Med 207, 2195–2206 (2010)
X. Wang, J. Wong, C.J. Sevinsky, L. Kokabee, F. Khan, Y. Sun, D.S. Conklin, Bruton’s tyrosine kinase inhibitors prevent therapeutic escape in breast cancer cells. Mol Cancer Ther 15, 2198–2208 (2016)
Y.H. Soung, T. Kashyap, T. Nguyen, G. Yadav, H. Chang, Y. Landesman, J. Chung, Selective Inhibitors of Nuclear Export (SINE) compounds block proliferation and migration of triple negative breast cancer cells by restoring expression of ARRDC3. Oncotarget 8, 52935 (2017)
S. Gessi, S. Bencivenni, E. Battistello, F. Vincenzi, V. Colotta, D. Catarzi, F. Varano, S. Merighi, P.A. Borea, K. Varani, Inhibition of A2A adenosine receptor signaling in cancer cells proliferation by the novel antagonist TP455. Front Pharmacol 8, 888 (2017)
A. McGray, Hallett R, Bernard D, et al., Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumour. Mol Ther 22, 206–218 (2014)
J. Plava, M. Cihova, M. Burikova, M. Matuskova, L. Kucerova, S. Miklikova, Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol Cancer 18, 1–10 (2019)
B. Afsane, Seyed Mahdi Hassanian, Majid Khazaei, et al., The therapeutic potential of targeting tumor microenvironment in breast cancer: rational strategies and recent progress, J Cell Biochem, (2017). https://doi.org/10.1002/jcb.26183
S. Soysal, Tzankov A, Muenst SE, Role of the tumor microenvironment in breast cancer, Pathobiology 82, 142-152 (2015)
B. Wolfson, Eades G, Zhou Q, Adipocyte activation of cancer stem cell signaling in breast cancer, World J Biol Chem 6, 39, (2015)
Q. Li, Xia J, Yao Y, Gong D-w, Shi H, Zhou Q, Sulforaphane inhibits mammary adipogenesis by targeting adipose mesenchymal stem cells, Breast Cancer Res Treat 141, 317-324 (2013)
A. Bondareva, Downey CM, Ayres F, et al., The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells, PloS One 4, e5620 (2009)
L. Chen, Tu SH, Huang CS, et al., Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway, Breast Cancer Res Treat 134, 989–1004 (2012)
C.J. Lovitt, T.B. Shelper, V.M. Avery, Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 18, 41 (2018)
H. Urakawa, Y. Nishida, J. Wasa, E. Arai, L. Zhuo, K. Kimata, E. Kozawa, N. Futamura, N. Ishiguro, Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int J Cancer 130, 454–466 (2012)
Z. Gong, M. Chen, Q. Ren, X. Yue, Z. Dai, Fibronectin-targeted dual-acting micelles for combination therapy of metastatic breast cancer. Signal Transduct Target Ther 5, 1–11 (2020)
K. Jiang, X. Song, L. Yang, L. Li, Z. Wan, X. Sun, T. Gong, Q. Lin, Z. Zhang, Enhanced antitumor and anti-metastasis efficacy against aggressive breast cancer with a fibronectin-targeting liposomal doxorubicin. J Control Release 271, 21–30 (2018)
A. Bai et al., GP369, an FGFR2-IIIb- specific antibody, exhibits potent antitumor activity against human cancers driven by activated FGFR2 signaling. Cancer Res 70, 7630–7639 (2010)
Y.K. Chae et al., Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget 8, 16052–16074 (2017)
A. Mitsuhashi et al., Fibrocyte- like cells mediate acquired resistance to anti- angiogenic therapy with bevacizumab. Nat Commun 6, 8792 (2015)
S. Biswas et al., Inhibition of TGF- beta with neutralizing antibodies prevents radiation- induced acceleration of metastatic cancer progression. J Clin Invest 117, 1305–1313 (2007)
K. Matsumoto, Nakamura T, Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer 119, 477–483 (2006)
K.-H. Kim, H. Kim, Progress of antibody-based inhibitors of the HGF–cMET axis in cancer therapy. Exp Mol Med 49, e307–e307 (2017)
Acknowledgements
Umar Mehraj is a recipient of a junior research fellowship (JRF) from UGC-CSIR, Govt. of India. Muzafar Ahmad Macha and Rais A. Ganai are recipients of a Ramanujan Fellowship from the Science & Engineering Research Board (SERB), Department of Science and Technology, Govt. of India, New Delhi. Figure 1 was designed using BioRender.com web resource.
Funding
The work was supported by a Ramalinga Swami Fellowship, Grant No. BT/HRD/35/02/2006, to Nissar Ahmad Wani from the Department of Biotechnology (DBT), Ministry of Science & Technology, New Delhi.
Author information
Authors and Affiliations
Contributions
N.A.W., M.A.M. (Manzoor A Mir) & UM initiated the study. U.M. M.A.M and N.A.W. collected the data, wrote the manuscript, and designed the figures and tables. R.A.G., M.A.M. (Muzafar A. Macha), A.H., M.A.Z., A.A.B., M.W.N., M.H., S.K.B. B.A., R.S.A.B, & M.A.M (Manzoor A Mir) critically revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehraj, U., Ganai, R.A., Macha, M.A. et al. The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: New challenges and therapeutic opportunities. Cell Oncol. 44, 1209–1229 (2021). https://doi.org/10.1007/s13402-021-00634-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-021-00634-9