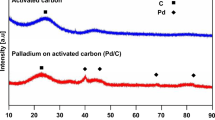
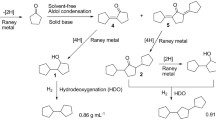
Abstract
Hydrogen transfer using a hydrogen donor solvent is promising for converting cellulose to liquid fuel. Alcohol and cyclic compounds are used as a hydrogen donor solvent, but the solvent affects the properties of liquid fuel. Therefore, the cellulose-derived liquid fuel and the solvent need to be separated. We focus on a straight-chain aliphatic hydrocarbon as a hydrogen source and solvent. Hydrogen transfer is expected by using palladium on activated catalyst (Pd/C) in combination with a solvent because palladium can dehydrogenate alkanes and is used as a hydrogenation metal. After the reaction, straight-chain aliphatic hydrocarbons remain in the cellulose-derived liquid fuel because they are similar to transportation fuel. Our previous study has reported that cellulose is converted into hydrocarbons by liquefaction using hexadecane containing Pd/C. However, the factors for deriving the optimum reaction conditions are unclear, such as the conversion route of cellulose to hydrocarbon and the formation mechanism of a solid component. In this study, we investigate the mechanism of the conversion of cellulose. The results indicate that the cellulose-derived oxygenates absorb on cellulose surface and form a solid component. In contrast, when Pd/C was added to tetradecane solvent, the oxygenates are hydrogenated to solvent-soluble compounds, resulting in suppressing the formation of solid components. The solvent-soluble compounds are deoxygenated to ketones, and then, the compounds changed to cyclopentanones and cyclohexanones which are the number of carbons > 6 at 330 °C because aldol condensation with the ketones occurs. Subsequently, the ketones are deoxygenated to hydrocarbons containing C10–C20 at 350 °C.

















Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Wei H, Liu W, Chen X, Yang Q, Li J, Chen H (2019) Renewable bio-jet fuel production for aviation: a review. Fuel 254(12):115599. https://doi.org/10.1016/j.fuel.2019.06.007
Wang WC, Tao L (2016) Bio-jet fuel conversion technologies. Renew Sustain Energy Rev 3:801–822. https://doi.org/10.1016/j.rser.2015.09.016
Cui X, Zhao X, Liu D (2018) A novel route for the flexible preparation of hydrocarbon jet fuels from biomass-based platform chemicals: a case of using furfural and 2,3-butanediol as feedstocks. Green Chem 9:2018–2026. https://doi.org/10.1039/C8GC00292D
Vânya CAS, Pasa MD (2019) Hydrogen-free process to convert lipids into bio-jet fuel and green diesel over niobium phosphate catalyst in one-step. Chem Eng J 370:98–109. https://doi.org/10.1016/j.cej.2019.03.063
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106(9):4044–4098. https://doi.org/10.1021/cr068360d
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311(5760):484–489. https://doi.org/10.1126/science.1114736
Czernik S, Bridgwater AV (2004) Overview of applications of biomass fast pyrolysis oil. Energy Fuels 18(2):590–598. https://doi.org/10.1021/ef034067u
Perkins G, Batalha N, Kumar A, Bhaskar T, Konarova M (2019) Recent advances in liquefaction technologies for production of liquid hydrocarbon fuels from biomass and carbonaceous wastes. Renew Sustain Energy Rev 115:109400. https://doi.org/10.1016/j.rser.2019.109400
Duan P, Savage PE (2011) Hydrothermal liquefaction of a microalga with heterogeneous catalysts. Ind Eng Chem Res 50(1):52–61. https://doi.org/10.1021/ie100758s
Elliott DC, Biller P, Ross AB, Schmidt AJ, Jones SB (2015) Hydrothermal liquefaction of biomass: developments from batch to continuous process. Biores Technol 178:147–156. https://doi.org/10.1016/j.biortech.2014.09.132
van Rossum G, Zhao W, Barnes MC, Lange JP, Kersten SRA (2014) Liquefaction of lignocellulosic biomass: solvent, process parameter, and recycle oil screening. Chemsuschem 7(1):253–259. https://doi.org/10.1002/cssc.201300297
Barnés MC, Oltvoort J, Kersten SRA, Lange JP (2017) Wood liquefaction: role of solvent. Ind Eng Chem Res 56(3):635–644. https://doi.org/10.1021/acs.iecr.6b04086
Wang X, Xie XA, Sun J, Liao W (2019) Effects of liquefaction parameters of cellulose in supercritical solvents of methanol, ethanol and acetone on products yield and compositions. Biores Technol 275:123–129. https://doi.org/10.1016/j.biortech.2018.12.047
Yuan XZ, Li H, Zeng GM, Tong JY, Xie W (2007) Sub- and supercritical liquefaction of rice straw in the presence of ethanol-water and 2-propanol-water mixture. Energy 32(11):2081–2088. https://doi.org/10.1016/j.energy.2007.04.011
Nielsen JB (2016) Valorization of lignin from biorefineries for fuels and chemicals. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark
Demirbaş A (2000) Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers Manage 41(6):633–646. https://doi.org/10.1016/S0196-8904(99)00130-2
Cheng S, Wei L, Julson J, Kharel PR, Cao Y, Gu Z (2017) Catalytic liquefaction of pine sawdust for biofuel development on bifunctional Zn/HZSM-5 catalyst in supercritical ethanol. J Anal Appl Pyrol 126:257–266. https://doi.org/10.1016/j.jaap.2017.06.001
Wang G, Li W, Li B, Chen H, Bai J (2007) Direct liquefaction of sawdust under syngas with and without catalyst. Chem Eng Process 46(3):187–192. https://doi.org/10.1016/j.cep.2006.05.014
Grilc M, Likozar B, Levec J (2016) Simultaneous liquefaction and hydrodeoxygenation of lignocellulosic biomass over NiMo/Al2O3, Pd/Al2O3, and zeolite Y catalysts in hydrogen donor solvents. ChemCatChem 8(1):180–191. https://doi.org/10.1002/cctc.201500840
Kakuta Y, Takiguchi K, Ishizu M, Ito T (2016) Additive effect of hydrogen donor on wood biomass liquefaction using diesel oil as a solvent. J Jpn Inst Energy 95(10):897–901. https://doi.org/10.3775/jie.95.897
Tsodikov MV, Chudakova MV, Chistyakov AV, Maksimov YV (2013) Catalytic conversion of cellulose into hydrocarbon fuel components. Pet Chem 53:367–373. https://doi.org/10.1134/S0965544113060145
Nawaz Z (2015) Light alkane dehydrogenation to light olefin technologies: a comprehensive review. Rev Chem Eng 31(5):413–436. https://doi.org/10.1515/revce-2015-0012
Kimura K, Niisaka S, Kakuta Y, Kurihara K (2020) Effect of hydrogen donation of palladium on active carbon on woody biomass pyrolysis using diesel oil as a solvent. J Jpn Inst Energy 99(1):8–15. https://doi.org/10.3775/jie.99.8
Kimura K, Saika Y, Kakuta Y, Kurihara K (2021) Catalytic transfer hydrogenation of cellulose to hydrocarbons using straight-chain aliphatic hydrocarbon as a solvent. Biomass Conv Bioref 11:873–884. https://doi.org/10.1007/s13399-020-01206-x
Fasolini A, Cucciniello R, Paone E, Mauriello F, Tabanelli TA (2019) Short overview on the hydrogen production via aqueous phase reforming (APR) of cellulose, C6–C5 Sugars and Polyols. Catalysts 9(11):917. https://doi.org/10.3390/catal9110917
Segal L, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786. https://doi.org/10.1177/004051755902901003
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J Appl Polym Sci 8:1325. https://doi.org/10.1002/app.1964.070080323
Alexander LE (1979) X-ray diffraction methods in polymer science. Krieger Publishing Co, New York
Scanlon JT, Willis DE (1985) Calculation of flame ionization detector relative response factors using the effective carbon number concept. J Chromatogr Sci 23(8):333–340. https://doi.org/10.1093/chromsci/36.5.223
Shafizadeh F, Furneaux RH, Cochran TG, Scholl JP, Sakai Y (1979) Production of levoglucosan and glucose from pyrolysis of cellulosic materials. J Appl Polym Sci 23:3525–3539. https://doi.org/10.1002/app.1979.070231209
Kwon GJ, Kim DY, Kimura S, Kuga S (2007) Rapid-cooling, continuous-feed pyrolyzer for biomass processing: preparation of levoglucosan from cellulose and starch. J Anal Appl Pyrol 80(1):1–5. https://doi.org/10.1016/j.jaap.2006.12.012
Kawamoto H, Saito S, Hatanaka W, Saka S (2007) Catalytic pyrolysis of cellulose in sulfolane with some acidic catalysts. J Wood Sci 53:127–133. https://doi.org/10.1007/s10086-006-0835-y
Yepez A, De S, Climent MS, Romero AA, Luque R (2015) Microwave-assisted conversion of levulinic acid to γ-valerolactone using low-loaded supported iron oxide nanoparticles on porous silicates. Appl Sci 5:532–543. https://doi.org/10.3390/app5030532
Amarasekara AS, Hasan MA (2015) Pd/C catalyzed conversion of levulinic acid to γ-valerolactone using alcohol as a hydrogen donor under microwave conditions. Catal Commun 60:5–7. https://doi.org/10.1016/j.catcom.2014.11.009
Wang A, Lu Y, Yi Z, Ashan E, Hu K, Zhang L, Yan K (2018) Selective production of γ-valerolactone and valeric acid in one-pot bifunctional metal catalysts. ChemistrySelect 3(4):1097–1101. https://doi.org/10.1002/slct.201702899
Pang SH, Medlin JW (2011) Adsorption and reaction of furfural and furfuryl alcohol on Pd (111): unique reaction pathways for multifunctional reagents. ACS Catal 1(10):1272–1283. https://doi.org/10.1021/cs200226h
Vorotnikov V, Mpourmpakis G, Vlachos DGDFT (2012) Study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on Pd (111). ACS Catal 2(12):2496–2504. https://doi.org/10.1021/cs300395a
Käldström M, Kumar N, Heikkilä T, Tiitta M, Salmi T, Murzin DYu (2011) Transformation of levoglucosan over H-MCM-22 zeolite and H-MCM-41 mesoporous molecular sieve catalysts. Biomass Bioenerg 35(5):1967–1976. https://doi.org/10.1016/j.biombioe.2011.01.046
Niu M, Hou Y, Ren S, Wang W, Zheng Q, Wu W (2015) The relationship between oxidation and hydrolysis in the conversion of cellulose in NaVO3-H2SO4 aqueous solution with O2. Green Chem 17:335–342. https://doi.org/10.1039/C4GC00970C
Licursi D, Antonetti C, Fulignati S, Giannoni M, Galletti AMR (2018) Cascade strategy for the tunable catalytic valorization of levulinic acid and γ-valerolactone to 2-methyltetrahydrofuran and alcohols. Catalysts 8:277. https://doi.org/10.3390/catal8070277
Sun D, Ohkubo A, Asami K, Katori T, Yamada Y, Sato S (2017) Vapor-phase hydrogenation of levulinic acid and methyl levulinate to γ-valerolactone over non-noble metal-based catalysts. Molecular Catalysis 437:105–113. https://doi.org/10.1016/j.mcat.2017.05.009
Koullas DP, Lois E, Koukios EG (1991) Effect of physical pretreatments on the prepyrolytic behaviour of lignocellulosics. Biomass Bioenerg 1(4):199–206. https://doi.org/10.1016/0961-9534(91)90003-U
Basch A, Lewin M (1973) The influence of fine structure on the pyrolysis of cellulose. II. Pyrolysis in air. Journal of Polymer Science: Polymer Chemistry Edition 11(12):3095–3101. https://doi.org/10.1002/pol.1973.170111205
Kawamoto H, Hatanaka W, Saka S (2003) Thermochemical conversion of cellulose in polar solvent (sulfolane) into levoglucosan and other low molecular-weight substances. J Anal Appl Pyrol 70(2):303–313. https://doi.org/10.1016/S0165-2370(02)00160-2
Kawamoto H, Saka S (2006) Heterogeneity in cellulose pyrolysis indicated from the pyrolysis in sulfolane. J Anal Appl Pyrol 76(1–2):280–284. https://doi.org/10.1016/j.jaap.2005.12.011
Agarwal V, Huber GW, Conner WC Jr, Auerbach SM (2011) Simulating infrared spectra and hydrogen bonding in cellulose Iβ at elevated temperatures. J Chem Phys 135:134506. https://doi.org/10.1063/1.3646306
Maréchala Y, Chanzy H (2000) The hydrogen bond network in Iβ cellulose as observed by infrared spectrometry. J Mol Struct 523(1–3):183–196. https://doi.org/10.1016/S0022-2860(99)00389-0
Liang CY, Marchessault RH (1959) Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native celluloses. J POLYMER SCI 37(132):385–395. https://doi.org/10.1002/pol.1959.1203713209
Pastorova I, Botto RE, Arisz PW, Boon JJ (1994) Cellulose char structure: a combined analytical Py-GC-MS, FTIR, and NMR study. Carbohyd Res 262(1):27–47. https://doi.org/10.1016/0008-6215(94)84003-2
Tang MM, Bacon R (1964) Carbonization of cellulose fibers-I. Low temperature pyrolysis Carbon 2(3):211–214. https://doi.org/10.1016/0008-6223(64)90035-1
Zheng Q, Morimoto M, Takanohashi T (2017) Production of carbonaceous microspheres from wood sawdust by a novel hydrothermal carbonization and extraction method. RSC Adv 7:42123–42128. https://doi.org/10.1039/C7RA07847A
Xu Z, Guo Z, Xiao X, Zeng P, Xue Q (2019) Effect of inorganic potassium compounds on the hydrothermal carbonization of Cd-contaminated rice straw for experimental-scale hydrochar. Biomass Bioenerg 130:105357. https://doi.org/10.1016/j.biombioe.2019.105357
Kumalaputri AJ, Randolph C, Otten E, Heeres HJ, Deuss PJ (2018) Lewis acid catalyzed conversion of 5-hydroxymethylfurfural to 1,2,4-benzenetriol, an overlooked biobased compound. ACS Sustainable Chem Eng 6(3):3419–3425. https://doi.org/10.1021/acssuschemeng.7b03648
Shangguan J, Pfriem N, Chin YH (2019) Mechanistic details of CO bond activation in and H-addition to guaiacol at water-Ru cluster interfaces. J Catal 370(186–199):S0021951718304779
Antonetti C, Maria A, Gallettia R, Accorinti P, Alini S, Babini P, Raabova K, Rozhko E, Caldarelli A, Righi P, Cavani F, Concepcion P (2013) Two alternative routes for 1,2-cyclohexanediol synthesis by means of green processes: cyclohexene dihydroxylation and catechol hydrogenation. Appl Catal A 466:21–31. https://doi.org/10.1016/j.apcata.2013.06.023
Zhang Y, Zhou J, Si J (2017) Synergistic catalysis of nano-Pd and nano rare-earth oxide/AC: complex nanostructured catalysts fabricated by a photochemical route for selective hydrogenation of phenol. RSC Adv 7:54779–54788. https://doi.org/10.1039/C7RA09917G
Luijkx GCA, van Rantwijk F, van Bekkum H (1993) Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and d-fructose. Carbohyd Res 242:131–139. https://doi.org/10.1016/0008-6215(93)80027-C
Wu Q, Zhao B, Liu S, Yu S, Huang L, Ragauskas AJ (2020) From cellulose to 1,2,4-benzenetriol via catalytic degradation over a wood-based activated carbon catalyst. Catal Sci Technol 10:3423–3432. https://doi.org/10.1039/D0CY00424C
Dohade M, Dhepe PL (2018) Efficient method for cyclopentanone synthesis from furfural: understanding the role of solvents and solubility in a bimetallic catalytic system. Catal Sci Technol 8:5259–5269. https://doi.org/10.1039/C8CY01468J
Hronec M, Fulajtárová K, Vávra I, Soták T, Dobročka E, Mičušík M (2016) Carbon supported Pd-Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl Catal B 181:210–219. https://doi.org/10.1016/j.apcatb.2015.07.046
Liu Y, Li G, Hu Y, Wang A, Lu F, Zou JJ, Cong Y, Li N, Zhang T (2019) Integrated conversion of cellulose to high-density aviation fuel. Joule 3(4):1028–1036. https://doi.org/10.1016/j.joule.2019.02.005
Bui TV, Sooknoi T, Resasco DE (2017) Simultaneous upgrading of furanics and phenolics through hydroxyalkylation/aldol condensation reactions. Chemsuschem 10:1–10. https://doi.org/10.1002/cssc.201601251
Luggren PJ, Di Cosimo JI (2020) Deactivation of Cu-Mg-Al mixed oxide catalysts for liquid transportation fuel synthesis from biomass-derived resources. Molecular Catalysis 481:110166. https://doi.org/10.1016/j.mcat.2018.08.008
Muldoon JA, Harvey BG (2020) Bio-based cycloalkanes: the missing link to high-performance sustainable jet fuels. Chemsuschem 13:5777–5807. https://doi.org/10.1002/cssc.202001641
Hirano Y, Miyata Y, Taniguchi M, Funakoshi N, Yamazaki Y, Ogino C, Kita Y (2020) Fe-assisted hydrothermal liquefaction of cellulose: effects of hydrogenation catalyst addition on properties of water-soluble fraction. J Analytic App Pyrolys 145:104719
Jin L, Li W, Liu Q, Ma L, Hu C, Ogunbiyi AT, Wu, Zhang MQ (2020) High performance of Mo-promoted Ir/SiO 2 catalysts combined with HZSM-5 toward the conversion of cellulose to C 5/C 6 alkanes. Bioresour Technol 297:122492
Xia Q, Chen Z, Shao Y, Gong X, Wang H, Liu X, Parker SF, Han X, Yang S, Wang Y (2016) Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat Commun 7:11162
Author information
Authors and Affiliations
Contributions
Kentaro Kimura, Yusuke Kakuta, and Kiyofumi Kurihara contributed to the conception or design of the work. Kentaro Kimura and Eri Watanabe helped in performing the catalytic reactions and analysis, and interpreted data for the study. All the authors debated the results and co-wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, K., Kakuta, Y., Watanabe, E. et al. Effect of formation behavior of hydrocarbons and solid component from cellulose on catalytic transfer hydrogenation in straight-chain aliphatic hydrocarbon solvent. Biomass Conv. Bioref. 13, 9903–9917 (2023). https://doi.org/10.1007/s13399-021-01823-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01823-0