Abstract
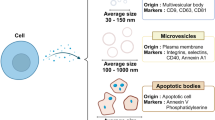
In recent years, different advancements have been observed in nanosized drug delivery systems. Factors such as stability, safety and targeting efficiency cause hindrances in the clinical translation of these synthetic nanocarriers. Therefore, researchers employed endogenous nanocarriers like exosomes as drug delivery vehicles that have an inherent ability to target more efficiently after appropriate functionalization and show higher biocompatibility and less immunogenicity and facilitate penetration through the biological barriers more quickly than the other available carriers. Exosomes are biologically derived lipid bilayer-enclosed nanosized extracellular vesicles (size ranges from 30 to 150 nm) secreted from both prokaryotic and eukaryotic cells and appears significantly in the extracellular space. These EVs (extracellular vesicles) can exist in different sources, including mammals, plants and microorganisms. Different advanced techniques have been introduced for the isolation of exosomes to overcome the existing barriers present with conventional methods. Extensive research on the application of exosomes in therapeutic delivery for treating various diseases related to central nervous system, bone, cancer, skin, etc. has been employed. Several studies are on different stages of clinical trials, and many exosomes patents have been registered.
Graphical abstract





Similar content being viewed by others
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Zhang M, Zang X, Wang M, Li Z, Qiao M, Hu H, et al. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: recent advances and challenges. J Mater Chem B. 2019;7:2421–33.
Ren J, He W, Zheng L, Duan H. From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomater Sci. 2016;4:910–21.
Jiang X, Gao J. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017. Available from: https://doi.org/10.1016/j.ijpharm.2017.02.038.
Suharta S, Indariani S, Ana ID. Plant-derived exosome-like nanoparticles : a concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. 2021;2838–50.
Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894–906. Available from: https://doi.org/10.1016/j.jconrel.2020.10.020.
Yuan L, Li J. Exosomes in Parkinson ’ s disease: current perspectives and future challenges. 2019.
Chen L, Wang L, Zhu L, Xu Z, Liu Y, Li Z, et al. Exosomes as drug carriers in anti-cancer therapy. Front Cell Dev Biol. 2022;10:1–12.
Mehryab F, Rabbani S, Shahhosseini S, Shekari F, Fatahi Y, Baharvand H, et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020. Available from: https://doi.org/10.1016/j.actbio.2020.06.036.
Donoso-Quezada J, Ayala-Mar S, González-Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics and applications. Traffic. 2021;22:204–20.
Betker JL, Angle BM, Graner MW, Anchordoquy TJ. The potential of exosomes from cow milk for oral delivery. J Pharm Sci. 2019;108:1496–505. Available from: https://doi.org/10.1016/j.xphs.2018.11.022.
Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their. Asian J Pharm Sci. 2022;17:53–69. Available from: https://doi.org/10.1016/j.ajps.2021.05.006.
Chen Q, Li Q, Liang Y, Zu M, Chen N, Canup BSB, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12:907–23. Available from: https://doi.org/10.1016/j.apsb.2021.08.016.
Gu Z, Li F, Liu Y, Jiang M, Zhang L, He L, et al. Through intestinal aryl hydrocarbon receptor in mice. 2021;5:846–64.
Jo CS, Myung CH, Yoon YC, Ahn BH, Min JW, Seo WS, et al. The effect of Lactobacillus plantarum extracellular vesicles from Korean women in their 20s on skin aging. Curr Issues Mol Biol. 2022;44:526–40.
Yoon YC, Ahn BH, Min JW, Lee KR, Park SH, Kang HC. Stimulatory effects of extracellular vesicles derived from Leuconostoc holzapfelii that exists in human scalp on hair growth in human follicle dermal papilla cells. Curr Issues Mol Biol. 2022;44:845–66.
Yang H, Ma Q, Wang Y, Tang Z. Clinical application of exosomes and circulating microRNAs in the diagnosis of pregnancy complications and foetal abnormalities. J Transl Med. 2020;18:1–9. Available from: https://doi.org/10.1186/s12967-020-02227-w.
Suh JH, Joo HS, Hong EB, Lee HJ, Lee JM. Therapeutic application of exosomes in inflammatory diseases. Int J Mol Sci. 2021;22:1–22.
Qiu H, Liu S, Wu K, Zhao R, Cao L, Wang H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: a review. J Cosmet Dermatol. 2020;19:574–81.
Li Y, Tang Y, Yang GY. Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc Neurol. 2021;6:483–95.
Han L, Zhao Z, Yang K, Xin M, Zhou L, Chen S, et al. Application of exosomes in the diagnosis and treatment of pancreatic diseases. Stem Cell Res Ther. 2022;13:1–11. Available from: https://doi.org/10.1186/s13287-022-02826-y.
Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16:24–40.
Mehryab F, Rabbani S, Shahhosseini S, Shekari F, Fatahi Y, Baharvand H, et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. Available from: https://doi.org/10.1016/j.actbio.2020.06.036.
Théry C, Duban L, Segura E, Væron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–62.
André F, Chaput N, Schartz NEC, Flament C, Aubert N, Bernard J, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic Cell-Derived Exosomes Transfer Functional MHC Class I/Peptide Complexes to Dendritic Cells. J Immunol. 2004;172:2126–36.
Hood JL, San Roman S, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–801.
Yeo RWY, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–41. Available from: https://doi.org/10.1016/j.addr.2012.07.001.
Mulcahy LA, Pink RC, Carter DRF. Biological agent-exosome compositions and uses thereof. J Extracell Vesicles. 2017;3.
Chung IM, Rajakumar G, Venkidasamy B, Subramanian U, Thiruvengadam M. Exosomes: current use and future applications. Clin Chim Acta. 2020;500:226–32. Available from: https://doi.org/10.1016/j.cca.2019.10.022.
Vlassov A V., Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta - Gen Subj. 2012;1820:940–8. Available from: https://doi.org/10.1016/j.bbagen.2012.03.017.
Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72:1095–102. Available from: https://doi.org/10.1038/sj.ki.5002486.
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–73.
Miranda KC, Bond DT, Mckee M, Skog J, Păunescu TG, Da SN, et al. Potential biomarkers for renal disease. 2015;78:191–9.
Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, et al. Exosomes: novel biomarkers for clinical diagnosis. Sci World J. 2015;2015.
Berckmans RJ, Sturk A, Van Tienen LM, Schaap MCL, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117:3172–80.
Turola E, Furlan R, Bianco F, Matteoli M, Verderio C. Microglial microvesicle secretion and intercellular signaling. Front Physiol. 2012;3 MAY:1–11.
Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27:31–9. Available from: https://doi.org/10.1016/j.blre.2012.12.002.
Yakubovich EI, Polischouk AG, Evtushenko VI. Principles and problems of exosome isolation from biological fluids. Biochem Suppl Ser A Membr Cell Biol. 2022;16:115–26.
Goh WJ, Zou S, Ong WY, Torta F, Alexandra AF, Schiffelers RM, et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7:1–10.
Isola AL, Chen S. Exosomes: the messengers of health and disease. Curr Neuropharmacol. 2016;15:157–65.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11. Available from: https://doi.org/10.1016/j.pharmthera.2018.02.013.
Garcia NA, Ontoria-Oviedo I, González-King H, Diez-Juan A, Sepúlveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS ONE. 2015;10:1–23.
Faught E, Henrickson L, Vijayan MM. Plasma exosomes are enriched in Hsp70 and modulated by stress and cortisol in rainbow trout. J Endocrinol. 2017;232:237–46.
Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Heidari Khoei H, Asghari MH. Immunoregulatory role of melatonin in cancer. J Cell Physiol. 2020;235:745–57.
Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29:13–31. Available from: https://doi.org/10.1016/j.ymthe.2020.11.030.
Chukhchin DG, Bolotova K, Sinelnikov I, Churilov D, Novozhilov E. Exosomes in the phloem and xylem of woody plants. Planta. 2020;251:1–14. Available from: https://doi.org/10.1007/s00425-019-03315-y.
Zhang Z, Yu Y, Zhu G, Zeng L, Xu S, Cheng H, et al. The emerging role of plant-derived exosomes-like nanoparticles in immune regulation and periodontitis treatment. Front Immunol. 2022;13:1–14.
Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17:53–69. Available from: https://doi.org/10.1016/j.ajps.2021.05.006.
Logozzi M, Di Raimo R, Mizzoni D, Fais S. The potentiality of plant-derived nanovesicles in human health—a comparison with human exosomes and artificial nanoparticles. Int J Mol Sci. 2022;23.
Nazimek K, Bryniarski K, Askenase PW. Functions of exosomes and microbial extracellular vesicles in allergy and contact and delayed-type hypersensitivity. Int Arch Allergy Immunol. 2016;171:1–26.
Fang Y, Wang Z, Liu X, Tyler BM. Biogenesis and biological functions of extracellular vesicles in cellular and organismal communication with microbes. Front Microbiol. 2022;13:1–15.
Wang J, Yao Y, Chen X, Wu J, Gu T, Tang X. Host derived exosomes-pathogens interactions: potential functions of exosomes in pathogen infection. Biomed Pharmacother. 2018;108:1451–9. Available from: https://doi.org/10.1016/j.biopha.2018.09.174.
Kulp A, Kuehn MJ. Functions and biogenesis of OMVs. Annu Rev Microbiol. 2010;64:163–84.
Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiol (United Kingdom). 2014;160:2109–21.
Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJP, Nosanchuk JD, et al. Biogenesis of extracellular vesicles in yeast. Commun Integr Biol. 2010;3:533–5.
Rizzo J, Taheraly A, Janbon G. Structure, composition and biological properties of fungal extracellular vesicles. microLife. 2021;2:1–13.
Rizzo J, Rodrigues ML, Janbon G. Extracellular vesicles in fungi: past, present, and future perspectives. Front Cell Infect Microbiol. 2020;10:1–13.
Moyano S, Musso J, Feliziani C, Zamponi N, Frontera LS, Ropolo AS, et al. Exosome biogenesis in the protozoa parasite Giardia lamblia: a model of reduced interorganellar crosstalk. Cells. 2019;8:1–19.
de Souza W. Exosomes in the pathogenic protozoan Trypanosoma cruzi. Int J Pathol Clin Res. 2017;3:1–9.
Lee J, Lee JH, Chakraborty K, Hwang J, Lee YK. Exosome-based drug delivery systems and their therapeutic applications. RSC Adv. 2022;12:18475–92.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. Available from: https://doi.org/10.1016/j.jconrel.2015.07.030.
Gutierrez-Millan C, Calvo Díaz C, Lanao JM, Colino CI. Advances in exosomes-based drug delivery systems. Macromol Biosci. 2021;21:1–19.
Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics. 2021;13:1–20.
Johnsen KB, Gudbergsson JM, Duroux M, Moos T, Andresen TL, Simonsen JB. On the use of liposome controls in studies investigating the clinical potential of extracellular vesicle-based drug delivery systems – a commentary. J Control Release. 2018;269:10–4. Available from: https://doi.org/10.1016/j.jconrel.2017.11.002.
Ratemi E, Sultana Shaik A, Al Faraj A, Halwani R. Alternative approaches for the treatment of airway diseases: focus on nanoparticle medicine. Clin Exp Allergy. 2016;46:1033–42.
Martin-Serrano Á, Gómez R, Ortega P, La MFJD. Nanosystems as vehicles for the delivery of antimicrobial peptides (Amps). Pharmaceutics. 2019;11:1–24.
Kučuk N, Primožič M, Knez Ž, Leitgeb M. Exosomes engineering and their roles as therapy delivery tools, therapeutic targets, and biomarkers. Int J Mol Sci. 2021;22.
Soliman HM, Ghonaim GA, Gharib SM, Chopra H, Farag AK, Hassanin MH, et al. Exosomes in Alzheimer’s disease: from being pathological players to potential diagnostics and therapeutics. Int J Mol Sci. 2021;22.
Rajput A, Varshney A, Bajaj R, Pokharkar V. Exosomes as new generation vehicles for drug delivery: biomedical applications and future perspectives. Molecules. 2022;27.
Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21:1–19.
Patil SM, Sawant SS, Kunda NK. Exosomes as drug delivery systems: a brief overview and progress update. Eur J Pharm Biopharm. 2020;154:259–69. Available from: https://doi.org/10.1016/j.ejpb.2020.07.026.
Böing AN, van der Pol E, Grootemaat AE, Coumans FAW, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3.
Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022;9:1–18.
US20170296626A1 - Exosome isolation - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/US20170296626A1/en?q=exosome&oq=exosome.
Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q, et al. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small. 2020;16:1–18.
Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques Theranostics. 2017;7:789–804.
Chen H, Wang L, Zeng X, Schwarz H, Nanda HS, Peng X, et al. Exosomes, a new star for targeted delivery. Front Cell Dev Biol. 2021;9.
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regen Med. 2021;18:499–511. Available from: https://doi.org/10.1007/s13770-021-00361-0.
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–49. Available from: https://doi.org/10.1016/j.biomaterials.2017.10.012.
Jia G, Han Y, An Y, Ding Y, He C, Wang X, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. Elsevier Ltd; 2018. Available from: https://doi.org/10.1016/j.biomaterials.2018.06.029.
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine Nanotechnology, Biol Med. 2018;14:195–204. Available from: https://doi.org/10.1016/j.nano.2017.09.011.
Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10:3323–33.
Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Control Release. 2020;317:273–81. Available from: https://doi.org/10.1016/j.jconrel.2019.11.009.
Choi ES, Song J, Kang YY, Mok H. Mannose-modified serum exosomes for the elevated uptake to murine dendritic cells and lymphatic accumulation. Macromol Biosci. 2019;19:1–10.
Dusoswa SA, Horrevorts SK, Ambrosini M, Kalay H, Paauw NJ, Nieuwland R, et al. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J Extracell Vesicles. 2019;8. Available from: https://doi.org/10.1080/20013078.2019.1648995.
Nakase I, Futaki S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep. 2015;5:1–13.
Tamura R, Uemoto S, Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–84. Available from: https://doi.org/10.1016/j.actbio.2017.05.013.
Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8:1869–78.
Wang J, Li W, Lu Z, Zhang L, Hu Y, Li Q, et al. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale. 2017;9:15598–605.
Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:1–11. Available from: https://doi.org/10.1038/srep21933.
Lee J, Lee H, Goh U, Kim J, Jeong M, Lee J, et al. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl Mater Interfaces. 2016;8:6790–5.
CA3082588A1 - Compositions of engineered exosomes and methods of loading luminal exosome payloads - Google Patents. [cited 2022 Sep 27]. Available from: https://patents.google.com/patent/CA3082588A1/en?q=Compositions+of+Engineered+Exosomes+and+Methods+of+Loading+Luminal+Exosome+Payloads&oq=Compositions+of+Engineered+Exosomes+and+Methods+of+Loading+Luminal+Exosome+Payloads.
US10561740B2 - Preparation of therapeutic exosomes using membrane proteins - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/US10561740B2/en?q=Preparation+of+therapeutic+exosome&oq=Preparation+of+therapeutic+exosome.
Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R, et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19–28.
Bai J, Duan J, Liu R, Du Y, Luo Q, Cui Y, et al. Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells. Asian J Pharm Sci. 2020;15:461–71. Available from: https://doi.org/10.1016/j.ajps.2019.04.002.
Bliss CM, Parsons AJ, Nachbagauer R, Hamilton JR, Cappuccini F, Ulaszewska M, et al. Targeting antigen to the surface of EVs improves the in vivo immunogenicity of human and non-human adenoviral vaccines in mice. Mol Ther - Methods Clin Dev. 2020;16:108–25. Available from: https://doi.org/10.1016/j.omtm.2019.12.003.
Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, et al. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28:536–47. Available from: https://doi.org/10.1016/j.ymthe.2019.11.020.
Sun C, Qin Y, Zhuang H, Zhang Y, Wu Z, Chen Y. Membrane vesicles as drug delivery systems: source, preparation, modification, drug loading, in vivo administration and biodistribution, and application in various diseases. Pharmaceutics. 2023;15.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Minimal information for studies of extracellular vesicles, et al. (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;2018:7.
Minimal information for studies of extracellular vesicles. (MISEV2022): a position statement of the International 2 Society for Extracellular Vesicles and update of the MISEV2018 guidelines. 3;2022
Khan SA, Rehman S, Nabi B, Iqubal A, Nehal N, Fahmy UA, et al. Boosting the brain delivery of Atazanavir through nanostructured lipid carrier-based approach for mitigating neuroaids. Pharmaceutics. 2020;12:1–26.
Logozzi M, Di Raimo R, Mizzoni D, Fais S. Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids. 1st ed. Methods Enzymol. Elsevier Inc. 2020. Available from: https://doi.org/10.1016/bs.mie.2020.06.011.
Xia Y, Chen T, Zhang L, Zhang X, Shi W, Chen G, et al. Colorimetric detection of exosomal microRNA through switching the visible-light-induced oxidase mimic activity of acridone derivate. Biosens Bioelectron. 2021;173:112834. Available from: https://doi.org/10.1016/j.bios.2020.112834.
US10557847B2 - Nano-plasmonic sensor for exosome detection - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/US10557847B2/en?q=exosome&oq=exosome.
Im H, Lee K, Weissleder R, Lee H, Castro CM. Novel nanosensing technologies for exosome detection and profiling. Lab Chip. 2017;17:2892–8.
Im H, Shao H, Weissleder R, Castro CM, Lee H. Nano-plasmonic exosome diagnostics. Expert Rev Mol Diagn. 2015;15:725–33.
Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, et al. Dopamine - loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–66.
Li Y, Wang G, Wang Q, Zhang Y, Cui L. Exosomes secreted from adipose-derived stem cells are a potential treatment agent for immune-mediated alopecia. J Immunol Res. 2022;2022:7471246.
Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, et al. Recent advancements in the use of exosomes as drug delivery systems 06 Biological Sciences 0601 Biochemistry and Cell Biology. J Nanobiotechnology. 2018;16:1–13. Available from: https://doi.org/10.1186/s12951-018-0403-9.
Lu CT, Zhao YZ, Wong HL, Cai J, Peng L, Tian XQ. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int J Nanomedicine. 2014;9:2241–57.
Liu L, Li Y, Peng H, Liu R, Ji W, Shi Z, et al. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing α-synuclein and immune activation of Parkinson’s disease. Sci Adv. 2020;6.
Cho E, Nam GH, Hong Y, Kim YK, Kim DH, Yang Y, et al. Comparison of exosomes and ferritin protein nanocages for the delivery of membrane protein therapeutics. J Control Release. 2018;279:326–35. Available from: https://doi.org/10.1016/j.jconrel.2018.04.037.
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–79.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033.
Chen YA, Lu CH, Ke CC, Chiu SJ, Jeng FS, Chang CW, et al. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer’s disease pathology and improve cognitive deficits. Biomedicines. 2021;9:1–19.
US20200171084A1 - Brain specific exosome based diagnostics and extracorporeal therapies - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/US20200171084A1/en?q=exosome&oq=exosome.
US11369634B2 - Exosome-based therapeutics against neurodegenerative disorders - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/US11369634B2/en?q=exosome&oq=exosome.
AU2010268367B2 - Exosome based treatment of cancer - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/AU2010268367B2/en?q=exosome&oq=exosome.
Kobayashi M, Sawada K, Miyamoto M, Shimizu A, Yamamoto M, Kinose Y, et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem Biophys Res Commun. 2020;527:153–61. Available from: https://doi.org/10.1016/j.bbrc.2020.04.076.
Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739–48. Available from: https://doi.org/10.1016/j.jhep.2017.05.019.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90. Available from: https://doi.org/10.1016/j.biomaterials.2013.11.083.
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, et al. Development of exosome – encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–64.
Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine Nanotechnology, Biol Med. 2018;14:1973–85. Available from: https://doi.org/10.1016/j.nano.2018.05.020.
Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261:118369. Available from: https://doi.org/10.1016/j.lfs.2020.118369.
EP3287137B1 - Method for disrupting exosomes, exosome disruption kit, and method for separating exosomes derived from normal cells - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/EP3287137B1/en?q=exosome&oq=exosome&page=3.
Wei F, Li M, Crawford R, Zhou Y, Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomate. 2019;86:480–92. Available from: https://doi.org/10.1016/j.actbio.2019.01.006.
Yan F, Zhong Z, Wang Y, Feng Y, Mei Z, Li H, et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnology. 2020;18:1–15. Available from: https://doi.org/10.1186/s12951-020-00675-6.
Tian H, Zhang T, Qin S, Huang Z, Zhou L, Shi J, et al. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J Hematol Oncol. BioMed Central; 2022. Available from: https://doi.org/10.1186/s13045-022-01320-5.
Li H, Feng Y, Zheng X, Jia M, Mei Z, Wang Y, Li C. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30. https://doi.org/10.1016/j.jconrel.2021.11.019.
Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil. 2016;24:2135–40. Available from: https://doi.org/10.1016/j.joca.2016.06.022.
Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12:36938–47.
Kim YS, Go G, Yun CW, Yea JH, Yoon S, Han SY, et al. Topical administration of melatonin-loaded extracellular vesicle-mimetic nanovesicles improves 2,4-dinitrofluorobenzene-induced atopic dermatitis. Biomolecules. 2021;11:1450.
Shi A, Li J, Qiu X, Sabbah M, Boroumand S, Huang TC, et al. Theranostics TGF- β loaded exosome enhances ischemic wound healing in vitro and in vivo. 2021;11:6616–31.
Nanobiotechnol J, Li X, Wang Y, Shi L, Li B, Li J, et al. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell ‑ derived iron oxide exosomes. J Nanobiotechnology. 2020;1–14. Available from: https://doi.org/10.1186/s12951-020-00670-x.
Dong X, Lei Y, Yu Z, Wang T, Liu Y, Han G, et al. Theranostics exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis. 2021;11:5107–26.
CN107007541B - Application of exosome in skin whitening preparation - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/CN107007541B/en?q=exosome&oq=exosome&page=2.
WO2017123022A1 - Stem cell-derived exosome containing high amount of growth factors - Google Patents. [cited 2022 Sep 28]. Available from: https://patents.google.com/patent/WO2017123022A1/en?q=exosome&oq=exosome&page=2.
Sun L, Fan M, Huang D, Li B, Xu R, Gao F, et al. Biomaterials clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. 2021;271.
Pozo-acebo L, M-c L, Hazas D, Tom J, Gil-cabrerizo P. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. 2021;22:1105.
Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–40.
Yong T, Zhang X, Bie N, Zhang H, Zhang X, Hakeem A, et al. Drug carriers for chemotherapy. Nat Commun. 2019; Available from: https://doi.org/10.1038/s41467-019-11718-4.
Lee GW, Sol H, Jong K, Baek S, Woon Y, Jeong J, et al. Exosome mediated transfer of miRNA ‐ 140 promotes enhanced chondrogenic differentiation of bone marrow stem cells for enhanced cartilage repair and regeneration. 2020;1–11.
Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, et al. Exosomes derived from miR ‐ 375 ‐ overexpressing human adipose mesenchymal stem cells promote bone regeneration. 2019;1–14.
Vashisht M, Rani P, Onteru SK, Singh D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl Biochem Biotechnol. 2017;183:993–1007.
Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. 2013;21:1345–57.
Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M, et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25:1641–54. Available from: https://doi.org/10.1016/j.ymthe.2017.01.025.
Heo JS. Selenium-stimulated exosomes enhance wound healing by modulating inflammation and angiogenesis. 2022;23:11543.
Jiang M. ThinkIR: The University of Louisville ’ s Institutional Repository Etracellular vesicles from Lactobacillus rhamnosus GG protect against alcohol-induced liver injury through suppression of intestinal MIR 194 and subsequent activation of FXR in mice. 2022;3822.
Safety and Tolerability Study of MSC Exosome Ointment - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT05523011?term=exosome&recrs=e&draw=2&rank=1.
Zhu YG, Shi M meng, Monsel A, Dai C xiang, Dong X, Shen H, et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res Ther. 2022;13:1–10.
Study investigating the ability of plant exosomes to deliver curcumin to normal and colon cancer tissue - Full Text View - ClinicalTrials.gov. [cited 2022 Oct 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT01294072?term=exosome&recrs=a&draw=1&rank=8.
Exosomes and Immunotherapy in non-Hodgkin B-cell lymphomas - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT03985696?term=exosome&recrs=a&draw=2&rank=15.
Allogenic mesenchymal stem cell derived exosome in patients with acute ischemic stroke - Full Text View - ClinicalTrials.gov. [cited 2022 Oct 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT03384433?term=exosome&recrs=a&draw=1&rank=2.
Application of Circulating exosomes in early diagnosis and prognosis evaluation after intracerebral hemorrhage - Full Text View - ClinicalTrials.gov. [cited 2022 Oct 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT05035134?term=exosome&recrs=a&draw=2&rank=7.
Immune modulation by exosomes in COVID-19 - Full Text View - ClinicalTrials.gov. [cited 2022 Oct 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT05191381?term=exosome&recrs=a&draw=2&rank=11.
Safety of injection of placental mesenchymal stem cell derived exosomes for treatment of resistant perianal fistula in Crohn’s patients - Full Text View - ClinicalTrials.gov. [cited 2022 Oct 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT05499156?term=exosome&recrs=d&draw=2&rank=1.
MSC-Exos promote healing of MHs - Full Text View - ClinicalTrials.gov. [cited 2022 Oct 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT03437759?term=exosome&recrs=d&draw=2&rank=4.
Intra-articular injection of MSC-derived exosomes in knee osteoarthritis (ExoOA-1) - Full Text View - ClinicalTrials.gov. [cited 2022 Oct 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT05060107?term=exosome&recrs=b&draw=2&rank=3.
Intra-discal injection of platelet-rich plasma (PRP) enriched with exosomes in chronic low back pain - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT04849429?term=exosome&recrs=e&draw=2&rank=3.
LRRK2 and other novel exosome proteins in Parkinson’s disease - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT01860118?term=exosome&recrs=e&draw=1&rank=11.
Evaluation of safety and efficiency of method of exosome inhalation in SARS-CoV-2 associated pneumonia. - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT04491240?term=exosome&recrs=e&draw=2&rank=6.
Trial of a vaccination with tumor antigen-loaded dendritic cell-derived exosomes - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT01159288?term=exosome&recrs=e&draw=2&rank=18.
Safety and efficacy of injection of human placenta mesenchymal stem cells derived exosomes for treatment of complex anal fistula - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT05402748?term=exosome&recrs=a&draw=2&rank=1.
A clinical study of mesenchymal progenitor cell exosomes nebulizer for the treatment of pulmonary infection - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT04544215?term=exosome&recrs=a&draw=2&rank=5.
Effect of UMSCs derived exosomes on dry eye in patients with cGVHD - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT04213248?term=exosome&recrs=a&draw=2&rank=19.
A phase II randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of exosomes overexpressing CD24 to prevent clinical deterioration in patients with moderate or severe COVID-19 infection - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT04969172?term=exosome&recrs=d&draw=2&rank=2.
Multicenter clinical research for early diagnosis of lung cancer using blood plasma derived exosome - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT04529915?term=exosome&recrs=d&draw=2&rank=3.
The pilot experimental study of the neuroprotective effects of exosomes in extremely low birth weight infants - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT05490173?term=exosome&recrs=b&draw=2&rank=1.
Exosome-based nanoplatform for Ldlr mRNA delivery in FH - Full Text View - ClinicalTrials.gov. [cited 2022 Sep 27]. Available from: https://clinicaltrials.gov/ct2/show/NCT05043181?term=exosome&recrs=b&draw=2&rank=5.
Batrakova EV, Kim MS. Development and regulation of exosome-based therapy products. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology. 2016;8:744–57.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–96. Available from: https://doi.org/10.1016/j.apsb.2016.02.001.
Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–43. Available from: https://www.sciencedirect.com/science/article/pii/S0168365922002905.
Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65:342–7. Available from: https://doi.org/10.1016/j.addr.2012.07.002.
Kibria G, Ramos EK, Wan Y, Gius DR, Liu H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol Pharm. 2018;15:3625–33.
Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41:835–42.
Acknowledgements
The authors wish to thank the DST FIST Govt. of India for providing infrastructural support to carry our research work.
Author information
Authors and Affiliations
Contributions
Conceptualization: AS; writing—original draft: HB, AS, DB, MD; writing—review and editing: HB, AS; supervision: AS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is a review-based study. It has been confirmed that no ethics approval is required.
Consent for publication
All authors have approved the content of this manuscript and have given full consent to submit this review for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baruah, H., Sarma, A., Basak, D. et al. Exosome: From biology to drug delivery. Drug Deliv. and Transl. Res. 14, 1480–1516 (2024). https://doi.org/10.1007/s13346-024-01515-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-024-01515-y