Abstract
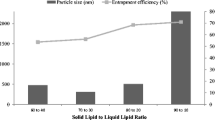
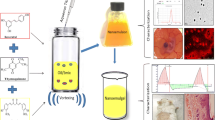
This study investigated a nanostructured lipid carrier (NLC)-gel system containing luteolin (LUT), a potential drug delivery system for the treatment of psoriasis. LUT-NLC was prepared by solvent emulsification ultrasonication method. The particle size was 199.9 ± 2.6 nm, with the encapsulation efficiency of 99.81% and drug loading of 4.06%. X-ray diffractometry (XRD), Fourier-transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC) were used to characterize the LUT-NLC. The NLC was dispersed in Carbomer 940 to form the NLC based gel. The rheological characteristics of LUT-NLC-gel showed an excellent shear-thinning behavior (non-Newtonian properties) and coincided with the Herschel-Bulkley model. LUT-NLC-gel (78.89 μg/cm2) exhibited better permeation properties and released over 36 hours than LUT gel (32.17 μg/cm2). The dye-labeled LUT-NLC presented intense fluorescence in the epidermis and dermis by the visualization of fluorescence and confocal microscopy, and it could accumulate in the hair follicles. The effect of LUT-NLC-gel on imiquimod-induced psoriasis mice was evaluated by psoriasis area severity index scoring, spleen index assay, histopathology, and inflammatory cytokines. These results confirmed that LUT-NLC-gel with high dose (80 mg/kg/day) remarkably reduced the level of inflammatory and proliferation factors such as TNF-α, IL-6, IL-17, and IL-23 in both skin lesions and blood. LUT-NLC-gel improved the macroscopic features. Therefore, the LUT-NLC-gel had great potential as an effective delivery system for skin diseases.
Graphical Abstract












Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available on reasonable request.
References
Wu S, Tian L. A new flavone glucoside together with known ellagitannins and flavones with anti-diabetic and anti-obesity activities from the flowers of pomegranate (Punica granatum). Nat Prod Res. 2019;33(2):252–7. https://doi.org/10.1080/14786419.2018.1446009.
Rodríguez-Calzada T, Qian M, Strid Å, et al. Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol Biochem. 2019;134:94–102. https://doi.org/10.1016/j.plaphy.2018.06.025.
Guenné S, Ouattara N, Ouédraogo N, Ciobica A, Hilou A, Kiendrebéogo M. Phytochemistry and neuroprotective effects of Eclipta alba (L.) Hassk. J Complement Integr Med. 2019;17(1):20190026. https://doi.org/10.1515/jcim-2019-0026.
Conti P, Caraffa A, Gallenga CE, et al. Powerful anti-inflammatory action of luteolin: Potential increase with IL-38. Biofactors. 2021;47(2):165–9. https://doi.org/10.1002/biof.1718.
Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian S. Autophagy regulation using luteolin: new insight into its anti-tumor activity. Cancer Cell Int. 2020;20(1):537. https://doi.org/10.1186/s12935-020-01634-9.
Manzoor MF, Ahmad N, Ahmed Z, et al. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J Food Biochem. 2019;43(9):e12974. https://doi.org/10.1111/jfbc.12974.
Schnarr L, Segatto ML, Olsson O, Zuin VG, Kümmerer K. Flavonoids as biopesticides - Systematic assessment of sources, structures, activities and environmental fate. Sci Total Environ. 2022;824:153781. https://doi.org/10.1016/j.scitotenv.2022.153781.
Singla RK, Kumar R, Khan S, Mohit Kumari K, Garg A. Natural Products: Potential Source of DPP-IV Inhibitors. Curr Protein Pept Sci. 2019;20(12):1218–25. https://doi.org/10.2174/1389203720666190502154129.
Kempuraj D, Thangavel R, Kempuraj DD, et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors. 2021;47(2):190–7. https://doi.org/10.1002/biof.1687.
Jiang D, Li D, Wu W. Inhibitory effects and mechanisms of luteolin on proliferation and migration of vascular smooth muscle cells. Nutrients. 2013;5(5):1648–59. https://doi.org/10.3390/nu5051648.
Ali F, Siddique YH. Bioavailability and Pharmaco-therapeutic Potential of Luteolin in Overcoming Alzheimer’s Disease. CNS Neurol Disord Drug Targets. 2019;18(5):352–65. https://doi.org/10.2174/1871527318666190319141835.
Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8(7):634–46. https://doi.org/10.2174/156800908786241050.
Imran M, Rauf A, Abu-Izneid T, et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother. 2019;112:108612. https://doi.org/10.1016/j.biopha.2019.108612.
Shakeel F, Haq N, Alshehri S, et al. Solubility, thermodynamic properties and solute-solvent molecular interactions of luteolin in various pure solvents. Journal of Molecular Liquids. 2018;255:43–50. https://doi.org/10.1016/j.molliq.2018.01.155.
McClements DJ. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol Adv. 2020;38:107287. https://doi.org/10.1016/j.biotechadv.2018.08.004.
Dobreva M, Stefanov S, Andonova V. Natural Lipids as Structural Components of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Topical Delivery. Curr Pharm Des. 2020;26(36):4524–35. https://doi.org/10.2174/1381612826666200514221649.
D’Souza A, Shegokar R. Nanostructured Lipid Carriers (NLCs) for Drug Delivery: Role of Liquid Lipid (Oil). Curr Drug Deliv. 2021;18(3):249–70. https://doi.org/10.2174/1567201817666200423083807.
Gendrisch F, Esser PR, Schempp CM, Wölfle U. Luteolin as a modulator of skin aging and inflammation. Biofactors. 2021;47(2):170–80. https://doi.org/10.1002/biof.1699.
Qing W, Wang Y, Li H, Ma F, Zhu J, Liu X. Preparation and Characterization of Copolymer Micelles for the Solubilization and In Vitro Release of Luteolin and Luteoloside. AAPS PharmSciTech. 2017;18(6):2095–101. https://doi.org/10.1208/s12249-016-0692-y.
Shimul Islam Md, Moshikur Rahman Md, Minamihata Kosuke, Moniruzzaman Muhammad, Kamiya N, Goto M. Choline oleate based micellar system as a new approach for Luteolin formulation: Antioxidant, antimicrobial, and food preservation properties evaluation. J Mol Liq. 2022;365:120151. https://doi.org/10.1016/j.molliq.2022.120151.
Gutiérrez RMP, Gómez JT, Urby RB, Soto JGC, Parra HR. Evaluation of Diabetes Effects of Selenium Nanoparticles Synthesized from a Mixture of Luteolin and Diosmin on Streptozotocin-Induced Type 2 Diabetes in Mice. Molecules. 2022;27(17):5642. https://doi.org/10.3390/molecules27175642.
Miyashita A, Ito J, Parida IS, et al. Improving water dispersibility and bioavailability of luteolin using microemulsion system. Sci Rep. 2022;12(1):11949. https://doi.org/10.1038/s41598-022-16220-4.
Elmowafy M, Shalaby K, Elkomy MH, et al. Development and assessment of phospholipid-based luteolin-loaded lipid nanocapsules for skin delivery. Int J Pharm. 2022;629:122375. https://doi.org/10.1016/j.ijpharm.2022.122375.
Liu Y, Wang L, Zhao Y, et al. Nanostructured lipid carriers versus microemulsions for delivery of the poorly water-soluble drug luteolin. Int J Pharm. 2014;476(1–2):169–77. https://doi.org/10.1016/j.ijpharm.2014.09.052.
Mahant S, Rao R, Souto EB, Nanda S. Analytical tools and evaluation strategies for nanostructured lipid carrier-based topical delivery systems. Expert Opin Drug Deliv. 2020;17(7):963–92. https://doi.org/10.1080/17425247.2020.1772750.
Weber S, Zimmer A, Pardeike J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22. https://doi.org/10.1016/j.ejpb.2013.08.013.
de Souza ML, Dos Santos WM, de Sousa ALMD, et al. Lipid Nanoparticles as a Skin Wound Healing Drug Delivery System: Discoveries and Advances. Curr Pharm Des. 2020;26(36):4536–50. https://doi.org/10.2174/1381612826666200417144530.
Rehman S, Nabi B, Baboota S, Ali J. Tailoring lipid nanoconstructs for the oral delivery of paliperidone: Formulation, optimization and in vitro evaluation. Chem Phys Lipids. 2021;234:105005. https://doi.org/10.1016/j.chemphyslip.2020.105005.
Shevalkar G, Pai R, Vavia P. Nanostructured Lipid Carrier of Propofol: a Promising Alternative to Marketed Soybean Oil-Based Nanoemulsion. AAPS PharmSciTech. 2019;20(5):201. https://doi.org/10.1208/s12249-019-1408-x.
Zingale E, Rizzo S, Bonaccorso A, et al. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics. 2022;14(9):1961. https://doi.org/10.3390/pharmaceutics14091961.
Rajpoot K, Prajapati SK, Malaiya A, Jain R, Jain A. Meropenem-Loaded Nanostructured Lipid Carriers For Skin and Soft Tissue Infection Caused by Staphylococcus aureus: Formulation, Design, and Evaluation. AAPS PharmSciTech. 2022;23(7):241. https://doi.org/10.1208/s12249-022-02381-y.
Hajipour H, Sambrani R, Ghorbani M, Mirzamohammadi Z, Nouri M. Sildenafil citrate-loaded targeted nanostructured lipid carrier enhances receptivity potential of endometrial cells via LIF and VEGF upregulation. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(11):2323–31. https://doi.org/10.1007/s00210-021-02153-8.
Bashyal S, Shin CY, Hyun SM, Jang SW, Lee S. Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl. Pharmaceutics. 2020;12(3):270. https://doi.org/10.3390/pharmaceutics12030270.
Parmar PK, Sharma N, Wasil Kabeer S, Rohit A, Bansal AK. Nanocrystal-based gel of apremilast ameliorates imiquimod-induced psoriasis by suppressing inflammatory responses. Int J Pharm. 2022;622:121873. https://doi.org/10.1016/j.ijpharm.2022.121873.
Sentjurc M, Vrhovnik K, Kristl J. Liposomes as a topical delivery system: the role of size on transport studied by the EPR imaging method. J Control Release. 1999;59(1):87–97. https://doi.org/10.1016/s0168-3659(98)00181-3.
Venturini CG, Bruinsmann FA, Contri RV, et al. Co-encapsulation of imiquimod and copaiba oil in novel nanostructured systems: promising formulations against skin carcinoma. Eur J Pharm Sci. 2015;79:36–43. https://doi.org/10.1016/j.ejps.2015.08.016.
Danaei M, Dehghankhold M, Ataei S, et al. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics. 2018;10(2):57. https://doi.org/10.3390/pharmaceutics10020057.
Ogiso T, Yamaguchi T, Iwaki M, Tanino T, Miyake Y. Effect of positively and negatively charged liposomes on skin permeation of drugs. J Drug Target. 2001;9(1):49–59. https://doi.org/10.3109/10611860108995632.
Niu H, Wang W, Dou Z, et al. Multiscale combined techniques for evaluating emulsion stability: A critical review. Adv Colloid Interface Sci. 2023;311:102813. https://doi.org/10.1016/j.cis.2022.102813.
Tesio AY, Robledo SN. Analytical determinations of luteolin. Biofactors. 2021;47(2):141–64. https://doi.org/10.1002/biof.1720.
Sun F, Li B, Guo Y, et al. Effects of ultrasonic pretreatment of soybean protein isolate on the binding efficiency, structural changes, and bioavailability of a protein-luteolin nanodelivery system. Ultrason Sonochem. 2022;88:106075. https://doi.org/10.1016/j.ultsonch.2022.106075.
Souto EB, Wissing SA, Barbosa CM, Müller RH. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur J Pharm Biopharm. 2004;58(1):83–90. https://doi.org/10.1016/j.ejpb.2004.02.015.
de Moura LD, Ribeiro LNM, de Carvalho FV, et al. Docetaxel and Lidocaine Co-Loaded (NLC-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma. Pharmaceutics. 2021;13(10):1552. https://doi.org/10.3390/pharmaceutics13101552.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl 1):S131–55. https://doi.org/10.1016/s0169-409x(02)00118-7.
Teeranachaideekul V, Boonme P, Souto EB, Müller RH, Junyaprasert VB. Influence of oil content on physicochemical properties and skin distribution of Nile red-loaded NLC. J Control Release. 2008;128(2):134–41. https://doi.org/10.1016/j.jconrel.2008.02.011.
Zhao J, Piao X, Shi X, Si A, Zhang Y, Feng N. Podophyllotoxin-Loaded Nanostructured Lipid Carriers for Skin Targeting: In Vitro and In Vivo Studies. Molecules. 2016;21(11):1549. https://doi.org/10.3390/molecules21111549.
Li B, Huang L, Lv P, et al. The role of Th17 cells in psoriasis. Immunol Res. 2020;68(5):296–309. https://doi.org/10.1007/s12026-020-09149-1.
van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–45. https://doi.org/10.4049/jimmunol.0802999.
Jemec GB, Wulf HC. The applicability of clinical scoring systems: SCORAD and PASI in psoriasis and atopic dermatitis. Acta Derm Venereol. 1997;77(5):392–3. https://doi.org/10.2340/0001555577392393.
Kim KE, Houh Y, Park HJ, Cho D. Therapeutic Effects of Erythroid Differentiation Regulator 1 on Imiquimod-Induced Psoriasis-Like Skin Inflammation. Int J Mol Sci. 2016;17(2):244. https://doi.org/10.3390/ijms17020244.
Hjuler KF, Gormsen LC, Vendelbo MH, Egeberg A, Nielsen J, Iversen L. Systemic Inflammation and Evidence of a Cardio-splenic Axis in Patients with Psoriasis. Acta Derm Venereol. 2018;98(4):390–5. https://doi.org/10.2340/00015555-2873.
Cheng Y, Liu Y, Tan J, et al. Spleen and thymus metabolomics strategy to explore the immunoregulatory mechanism of total withanolides from the leaves of Datura metel L. on imiquimod-induced psoriatic skin dermatitis in mice. Biomed Chromatogr. 2020;34(9):e4881. https://doi.org/10.1002/bmc.4881.
Kubin ME, Kokkonen N, Palatsi R, et al. Clinical Efficiency of Topical Calcipotriol/Betamethasone Treatment in Psoriasis Relies on Suppression of the Inflammatory TNFα - IL-23 - IL-17 Axis. Acta Derm Venereol. 2017;97(4):449–55. https://doi.org/10.2340/00015555-2579.
Khueangchiangkhwang S, Wu Z, Nagano I, Maekawa Y. Trichinella pseudospiralis-secreted 53 kDa protein ameliorates imiquimod-induced psoriasis by inhibiting the IL-23/IL-17 axis in mice. Biochem Biophys Rep. 2022;33:101415. https://doi.org/10.1016/j.bbrep.2022.101415.
Acknowledgments
Thanks Dr. Jinghan Li for the suggestion of the experiments.
Funding
This project is funded by Livelihood Plan Project of Department of Science and Technology of Liaoning Province (2021JH2/10300069, 2019-ZD-0845); Department of Education of Liaoning Province (LJKZ0918); and National College Students' innovation and entrepreneurship training program (202210163013).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hongjia Xu, Hao Hu and Mengyuan Zhao. Caihong Shi was supervised in the study. The first draft of the manuscript was written by Hongjia Xu and all authors commented on previous versions of the manuscript. Xiangrong Zhang wrote, reviewed and edited the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical statement
The study was reviewed and approved by the Institutional Animal Ethical Committee with registration number SYPHU-IACUC-S203-0216-103.
Consent for publication
The manuscript was not contained any individual person’ s data in any form (including any individual details, images or videos).
Conflict of interest
Author Hongjia Xu, Author Hao Hu, Author Mengyuan Zhao, Caihong Shi and Xiangrong Zhang declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Hu, H., Zhao, M. et al. Preparation of luteolin loaded nanostructured lipid carrier based gel and effect on psoriasis of mice. Drug Deliv. and Transl. Res. 14, 637–654 (2024). https://doi.org/10.1007/s13346-023-01418-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01418-4