Abstract
Purpose
Clearance of brain waste in the cerebrospinal fluid (CSF) through the meningeal lymphatic vessels (mLV) has been evaluated mostly through the fluorescent imaging which has inherent limitations in the context of animal physiology and clinical translatability. The study aimed to establish molecular imaging for the evaluation of mLV clearance function.
Methods
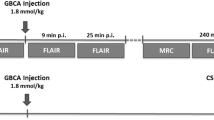
Radionuclide imaging after intrathecal (IT) injection was acquired in C57BL/6 mice of 2–9 months. The distribution of [99mTc]Tc-diethylenetriamine pentaacetate (DTPA) and [64Cu]Cu-human serum albumin (HSA) was comparatively evaluated. Evans Blue and [64Cu]Cu-HSA were used to evaluate the distribution of tracer under various speed and volume conditions.
Results
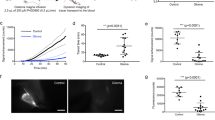
[99mTc]Tc-DTPA is not a suitable tracer for evaluation of CSF clearance via mLV as no cervical lymph node uptake was observed while it was cleared from the body. A total volume of 3 to 9 μL at an infusion rate of 300 to 500 nL/min was not sufficient for the tracer to reach the cranial subarachnoid space and clear throughout the mLV. As a result, whole-body positron emission tomography imaging using [64Cu]Cu-HSA at 700 nL/min, to deliver 6 μL of injected volume, was set for characterization of the CSF to mLV clearance. Through this protocol, the mean terminal CSF clearance half-life was measured to be 123.6 min (range 117.0–135.0) in normal mice.
Conclusions
We established molecular imaging to evaluate CSF drainage through mLV using [64Cu]Cu-HSA. This imaging method is expected to be extended in animal models of dysfunctional meningeal lymphatic clearance and translational research for disease-modifying therapeutic approaches.





Similar content being viewed by others
Data Availability
The data and material can be made be available upon communication with the corresponding author.
References
Ding J, Ji J, Rabow Z, Shen T, Folz J, Brydges CR, et al. A metabolome atlas of the aging mouse brain. Nat Commun. 2021;12:6021.
Panyard DJ, Kim KM, Darst BF, Deming YK, Zhong X, Wu Y, et al. Cerebrospinal fluid metabolomics identifies 19 brain-related phenotype associations. Commun Biol. 2021;4:63.
Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–8.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41.
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–9.
Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6.
Bucchieri F, Farina F, Zummo G, Cappello F. Lymphatic vessels of the dura mater: a new discovery? J Anat. 2015;227:702–3.
Sandrone S, Moreno-Zambrano D, Kipnis J, van Gijn J. A (delayed) history of the brain lymphatic system. Nat Med. 2019;25:538–40.
Lee DS, Suh M, Sarker A, Choi Y. Brain glymphatic/lymphatic imaging by MRI and PET. Nucl Med Mol Imaging. 2020;54:207–23.
Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100:375–88.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4.
Nakada T, Kwee IL. Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist. 2019;25:155–66.
Castro Dias M, Mapunda JA, Vladymyrov M, Engelhardt B. Structure and junctional complexes of endothelial, epithelial and glial brain barriers. Int J Mol Sci. 2019;20.
Kutomi O, Takeda S. Identification of lymphatic endothelium in cranial arachnoid granulation-like dural gap. Microscopy (Oxf). 2020;69:391–400.
Kido DK, Gomez DG, Pavese AM Jr, Potts DG. Human spinal arachnoid villi and granulations. Neuroradiology. 1976;11:221–8.
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–91.
Frederick N, Louveau A. Meningeal lymphatics, immunity and neuroinflammation. Curr Opin Neurobiol. 2020;62:41–7.
Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–6.
Wang L, Zhang Y, Zhao Y, Marshall C, Wu T, Xiao M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 2019;29:176–92.
Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–25.
Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17:1016–24.
Lee Y, Choi Y, Park EJ, Kwon S, Kim H, Lee JY, et al. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model. Sci Rep. 2020;10:16144.
Ma D, Holmes HE, Cardoso MJ, Modat M, Harrison IF, Powell NM, et al. Study the longitudinal in vivo and cross-sectional ex vivo brain volume difference for disease progression and treatment effect on mouse model of tauopathy using automated MRI structural parcellation. Front Neurosci. 2019;13:11.
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–309.
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–93.
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–61.
Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife. 2017;6.
Wu PC, Shieh DB, Hsiao HT, Wang JC, Lin YC, Liu YC. Magnetic field distribution modulation of intrathecal delivered ketorolac iron-oxide nanoparticle conjugates produce excellent analgesia for chronic inflammatory pain. J Nanobiotechnology. 2018;16:49.
Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5.
Wang X, Lou N, Eberhardt A, Yang Y, Kusk P, Xu Q, et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med. 2020;12.
Moazen M, Alazmani A, Rafferty K, Liu ZJ, Gustafson J, Cunningham ML, et al. Intracranial pressure changes during mouse development. J Biomech. 2016;49:123–6.
Rudick RA, Zirretta DK, Herndon RM. Clearance of albumin from mouse subarachnoid space: a measure of CSF bulk flow. J Neurosci Methods. 1982;6:253–9.
Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J. 2005;19:76–8.
Boulton M, Armstrong D, Flessner M, Hay J, Szalai JP, Johnston M. Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol. 1998;275:R889–96.
Patel M, Atyani A, Salameh JP, McInnes M, Chakraborty S. Safety of intrathecal administration of gadolinium-based contrast agents: a systematic review and meta-analysis. Radiology. 2020;297:75–83.
Eide PK, Vatnehol SAS, Emblem KE, Ringstad G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep. 2018;8.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7.
Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab. 2017;37:2112–24.
Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. 2020;367.
Li D, Li Y, Tian Y, Xu Z, Guo Y. Direct intrathecal injection of recombinant adeno-associated viruses in adult mice. J Vis Exp. 2019.
Yao L, Xue X, Yu P, Ni Y, Chen F. Evans Blue dye: a revisit of its applications in biomedicine. Contrast Media Mol Imaging. 2018;2018:7628037.
Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, et al. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS. 2012;9:3.
Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;11:107.
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–9.
Belov V, Appleton J, Levin S, Giffenig P, Durcanova B, Papisov M. Large-volume intrathecal administrations: impact on CSF pressure and safety implications. Front Neurosci. 2021;15:604197.
Linder MC, Wooten L, Cerveza P, Cotton S, Shulze R, Lomeli N. Copper transport. Am J Clin Nutr. 1998;67:965S–71S.
Ito S, Fujita H, Narita T, Yaginuma T, Kawarada Y, Kawagoe M, et al. Urinary copper excretion in type 2 diabetic patients with nephropathy. Nephron. 2001;88:307–12.
Biceroglu H, Albayram S, Ogullar S, Hasiloglu ZI, Selcuk H, Yuksel O, et al. Direct venous spinal reabsorption of cerebrospinal fluid: a new concept with serial magnetic resonance cisternography in rabbits. J Neurosurg Spine. 2012;16:394–401.
Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. 2020;11:1–9.
Novotny C, Potzi C, Asenbaum S, Peloschek P, Suess E, Hoffmann M. SPECT/CT fusion imaging in radionuclide cisternography for localization of liquor leakage sites. J Neuroimaging. 2009;19:227–34.
Verma A, Hesterman JY, Chazen JL, Holt R, Connolly P, Horky L, et al. Intrathecal (99m)Tc-DTPA imaging of molecular passage from lumbar cerebrospinal fluid to brain and periphery in humans. Alzheimers Dement (Amst). 2020;12:e12030.
Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–83.
Hirokawa N, Shiomura Y, Okabe S. Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol. 1988;107:1449–59.
Flach K, Hilbrich I, Schiffmann A, Gartner U, Kruger M, Leonhardt M, et al. Tau oligomers impair artificial membrane integrity and cellular viability. J Biol Chem. 2012;287:43223–33.
Roman AY, Devred F, Byrne D, La Rocca R, Ninkina NN, Peyrot V, et al. Zinc induces temperature-dependent reversible self-assembly of tau. J Mol Biol. 2019;431:687–95.
Rao DA, Forrest ML, Alani AW, Kwon GS, Robinson JR. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J Pharm Sci. 2010;99:2018–31.
Nakamura T, Kawai M, Sato Y, Maeki M, Tokeshi M, Harashima H. The effect of size and charge of lipid nanoparticles prepared by microfluidic mixing on their lymph node transitivity and distribution. Mol Pharm. 2020;17:944–53.
Russell CD, Crittenden RC, Cash AG. Determination of net ionic charge on Tc-99m DTPA and Tc-99m EDTA by a column ion-exchange method. J Nucl Med. 1980;21:354–60.
Alvarez B, Carballal S, Turell L, Radi R. Formation and reactions of sulfenic acid in human serum albumin. Methods Enzymol. 2010;473:117–36.
Wegmann S, Biernat J, Mandelkow E. A current view on tau protein phosphorylation in Alzheimer’s disease. Curr Opin Neurobiol. 2021;69:131–8.
Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, et al. Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Front Cell Neurosci. 2018;12:338.
Tepper K, Biernat J, Kumar S, Wegmann S, Timm T, Hubschmann S, et al. Oligomer formation of tau protein hyperphosphorylated in cells. J Biol Chem. 2014;289:34389–407.
Park JY, Song MG, Kim WH, Kim KW, Lodhi NA, Choi JY, et al. Versatile and finely tuned albumin nanoplatform based on click chemistry. Theranostics. 2019;9:3398–409.
Low BE, Wiles MV. A humanized mouse model to study human albumin and albumin conjugates pharmacokinetics. Methods Mol Biol. 2016;1438:115–22.
Britton S, Celada F. Immunogenicity of human serum albumin: decay in the normal mouse. Immunology. 1968;14:503–9.
Lei HY, Lee SH, Leir SH. Antigen-induced anaphylactic death in mice. Int Arch Allergy Immunol. 1996;109:407–12.
Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz J-L, Emery E, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45:3092–6.
Jacob L, Boisserand LSB, Geraldo LHM, de Brito NJ, Mathivet T, Antila S, et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun. 2019;10:4594.
Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11.
Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8.
Xue Y, Liu X, Koundal S, Constantinou S, Dai F, Santambrogio L, et al. In vivo T1 mapping for quantifying glymphatic system transport and cervical lymph node drainage. Sci Rep. 2020;10:14592.
Papisov MI, Belov VV, Gannon KS. Physiology of the intrathecal bolus: the leptomeningeal route for macromolecule and particle delivery to CNS. Mol Pharm. 2013;10:1522–32.
Du Boulay G, O'Connell J, Currie J, Bostick T, Verity P. Further investigations on pulsatile movements in the cerebrospinal fluid pathways. Acta Radiol Diagn (Stockh). 1972;13:496–523.
Matsumae M, Kuroda K, Yatsushiro S, Hirayama A, Hayashi N, Takizawa K, et al. Changing the currently held concept of cerebrospinal fluid dynamics based on shared findings of cerebrospinal fluid motion in the cranial cavity using various types of magnetic resonance imaging techniques. Neurol Med Chir (Tokyo). 2019;59:133–46.
Ma Q, Decker Y, Müller A, Ineichen BV, Proulx ST. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J Exp Med. 2019;216:2492–502.
Bachmann SB, Proulx ST, He Y, Ries M, Detmar M. Differential effects of anaesthesia on the contractility of lymphatic vessels in vivo. J Physiol. 2019;597:2841–52.
Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–7.
Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277:1127–34.
Mentis AA, Dardiotis E, Chrousos GP. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease: a conceptual framework. Mol Psychiatry. 2021;26:1075–97.
Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, et al. Development and plasticity of meningeal lymphatic vessels. J Exp Med. 2017;214:3645–67.
Li MN, Jing YH, Wu C, Li X, Liang FY, Li G, et al. Continuous theta burst stimulation dilates meningeal lymphatic vessels by up-regulating VEGF-C in meninges. Neurosci Lett. 2020;735:135197.
Machhi J, Yeapuri P, Lu Y, Foster E, Chikhale R, Herskovitz J, et al. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J Neuroinflammation. 2021;18:272.
Wyatt-Johnson SK, Brutkiewicz RR. The complexity of microglial interactions with innate and adaptive immune cells in Alzheimer’s disease. Front Aging Neurosci. 2020;12.
Freesmeyer M, Schwab M, Besteher B, Gröber S, Waschke A, Drescher R. High-resolution PET cisternography with 64Cu-DOTA for CSF leak detection. Clin Nucl Med. 2019;44:735–7.
Schievink WI. Spontaneous intracranial hypotension. N Engl J Med. 2021;385:2173–8.
Schievink WI, Maya MM, Moser F, Prasad R, Wadhwa V, Cruz R, et al. Multiple spinal CSF leaks in spontaneous intracranial hypotension: do they exist? Neurol Clin Pract. 2021;11:e691–e7.
Nicholson PJ, Guest WC, van Prooijen M, Farb RI. Digital subtraction myelography is associated with less radiation dose than CT-based techniques. Clin Neuroradiol. 2021;31:627–31.
Kim DK, Brinjikji W, Morris PP, Diehn FE, Lehman VT, Liebo GB, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol. 2020;41:21–8.
Hablitz LM, Pla V, Giannetto M, Vinitsky HS, Staeger FF, Metcalfe T, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11:4411.
Acknowledgements
Youngjoo Kim and Shin Jin Seop for their technical assistance.
Author information
Authors and Affiliations
Contributions
The first, second, third, ninth, and last authors were responsible for the study design. The first and second authors were responsible for interpretation of data and manuscript drafting. The first eight authors were also responsible for performing experiments and data documentation.
Corresponding authors
Ethics declarations
Competing Interests
Azmal Sarker was supported by SNU Presidential Fellowship. Minseok Suh, Yoori Choi, Ji Yong Park, Hyeyeon Seo, Seokjun Kwon, Hyun Kim, Eunji Lee, and Dong Soo Lee declares that they have no conflict of interest.
Ethics Approval and Consent to Participate
The study and experiment methodology of this study were approved by the Institutional Review Board and Institutional Animal Care and Use Committee (registration number SNU-200513-8) of Seoul National University College of Medicine. All experiments were conducted under relevant guidelines and regulations regarding the care and the use of animals for the experimental procedures.
Consent for Publication
None
Conflict of Interest
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2020R1A2C2101069 and No. 2021R1F1A1064340).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarker, A., Suh, M., Choi, Y. et al. [64Cu]Cu-Albumin Clearance Imaging to Evaluate Lymphatic Efflux of Cerebrospinal Space Fluid in Mouse Model. Nucl Med Mol Imaging 56, 137–146 (2022). https://doi.org/10.1007/s13139-022-00746-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-022-00746-6