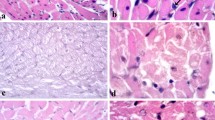
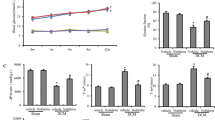
Abstract
Diabetes is a risk factor for cardiovascular disease that has a multifactorial etiology, with oxidative stress as an important component. Our previous observation of a significant diabetes-related increase in rat cardiac catalase (CAT) activity suggested that CAT could play a major role in delaying the development of diabetic cardiomyopathy. Thus, in the present work, we examined the effects of the daily administration of the CAT inhibitor, 3-amino-1,2,4-triazole (1 mg/g), on the hearts of streptozotocin (STZ)-induced diabetic rats. Administration of CAT inhibitor was started from the 15th day after the last STZ treatment (40 mg/kg/5 days), and maintained until the end of the 4th or 6th weeks of diabetes. Compared to untreated diabetic rats, at the end of the observation period, CAT inhibition lowered the induced level of cardiac CAT activity to the basal level and decreased CAT protein expression, mediated through a decline in the nuclear factor erythroid-derived 2-like 2 /nuclear factor-kappa B p65 (Nrf2/NF-κB p65) subunit ratio. The perturbed antioxidant defenses resulting from CAT inhibition promoted increased H2O2 production (P < 0.05) and lipid peroxidation (P < 0.05). Generated cytotoxic stimuli increased DNA damage (P < 0.05) and activated pro-apoptotic events, observed as a decrease (P < 0.05) in the ratio of the apoptosis regulator proteins Bcl-2/Bax, increased (P < 0.05) presence of the poly(ADP-ribose) polymerase-1 (PARP-1) 85 kDa apoptotic fragment and cytoplasmic levels of cytochrome C. These findings confirm an important function of CAT in the suppression of events leading to diabetes-promoted cardiac dysfunction and cardiomyopathy.





Similar content being viewed by others
References
Ahmed D, Kumar V, Verma A, Gupta PS, Kumar H, Dhingra V, Mishra V, Sharma M (2014) Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement Alternat Med 14:243–259
Ansley DM, Wang B (2013) Oxidative stress and myocardial injury in the diabetic heart. J Pathol 229:232–241
Aronson D (2003) Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21:3–12
Asbun J, Villarreal FJ (2006) The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 47:693–700
Asghar O, Al-Sunni A, Khavandi K, Khavandi A, Withers S, Greenstein A, Heagerty AM, Malik RA (2009) Diabetic cardiomyopathy. Clin Sci 116:741–760
Bagnyukova TV, Storey KB, Lushchak VI (2005) Adaptive response of antioxidant enzymes to catalase inhibition by aminotriazole in goldfish liver and kidney. Comp Biochem Physiol B 142:335–341
Basu A, Haldar S (1998) The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod 4:1099–1109
Beutler E (1982) Catalase. In: Beutler E (ed) Red cell metabolism: a manual of biochemical methods. Grune and Stratton, New York, pp 105–106
Bukan N, Sancak B, Yavuz O, Koca C, Tutkun F, Ozcelikay AT, Altan N (2003) Lipid peroxidation and scavenging enzyme levels in the liver of streptozotocin-induced diabetic rats. Indian J Biochem Biophys 40:447–450
Cai L, Kang YJ (2003) Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol 3:219–228
Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ (2006) Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 48:1688–1697
Ceriello A (2003) New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care 26:1589–1596
Ceriello A, Morocutti A, Mercuri L, Quagliaro L, Moro M, Damante G, Viberti GC (2000) Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes 49:2170–2177
D’Amours D, Sallmann FR, Dixit VM, Poirier GG (2001) Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci 114:3771–3778
Dreger H, Westphal K, Weller A, Baumann G, Stangl V, Meiners S, Stangl K (2009) Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res 83:354–361
Egorova MV, Afanasèv SA, Popov SV (2005) A simple method for isolation of cardiomyocytes from adult rat heart. Bull Exp Biol Med 140:370–373
Fang ZY, Prins JB, Marwick T (2004) Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 25:543–567
Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P (2000) Myocardial cell death in human diabetes. Circ Res 87:1123–1132
Gechev TS, Hille J (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 168:17–20
Grimm S, Bauer M, Baeuerle P, Schulze-Osthoff K (1996) Bcl-2 down-regulates the activity of transcription factor NF-kB induced upon apoptosis. J Cell Biol 134:13–23
Habig WH, Pabst MJ, Jakoby WB(1974) Gluthathione S-transferases: The first enzymatic step in mercapturic acid formation.. J Biol Chem 249:7130–7139
Hayat SA, Patel B, Khattar RS, Malik RA (2004) Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci 107:539–557
He X, Ma Q (2012) Disruption of Nrf2 synergizes with high glucose to cause heightened myocardial oxidative stress and severe cardiomyopathy in diabetic mice. J Diabetes Metab S7:002. doi:10.4172/2155-6156.S7-002
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. In: Storey KB (ed) Functional metabolism: regulation and adaptation. Wiley-Liss, Hoboken, pp 319–368
Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P (2003) Control of Bcl-2 expression by reactive oxygen species. PNAS 100:15035–15040
Hou Q, Hsu YT (2005) Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis: development of a GFP-Bax-stable H9c2 cell line for apoptosis analysis. Am J Physiol Heart Circ Physiol 289:H477–H487
Huang H, Shan J, Pan XH, Wang HP, Qian LB (2006) Carvedilol protected diabetic rat hearts via reducing oxidative stress. J Zhejiang Univ Sci B 7:725–731
Ivanović-Matić S, Mihailović M, Dinić S, Martinović V, Bogojević D, Grigorov I, Poznanovic G (2010) The absence of cardiomyopathy is accompanied by increased activities of CAT, MnSOD and GST in long-term diabetes in rats. J Physiol Sci 60:259–266
Izawa S, Inoue Y, Kimura A (1996) Importance of catalase in the adaptive response to hydrogen peroxide: analysis of acatalasaemic Saccharomyces cerevisiae. Biochem J 320:61–67
Kumar S, Prasad S, Sitasawad SL (2013) Multiple antioxidants improve cardiac complications and inhibit cardiac cell death in streptozotocin-induced diabetic rats. PLoS ONE 8:e67009. doi:10.1371/journal.pone.0067009
Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ (1995) Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11:376–381
Margoliash E, Novogrodsky A, Schejter A (1960) Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J 74:339–348
Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J (2010) NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85:473–483
Maritim AC, Sanders RA, Watkins JB (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
Matés JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Miao L, St-Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47:344–356
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Narula J, Kolodgie FD, Virmani R (2000) Apoptosis and cardiomyopathy. Curr Opin Cardiol 15:183–188
Ohkawa H, Okishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pedruzzi LM, Stockler-Pinto MB, Leite M Jr, Mafra D (2012) Nrf2-keap1 system versus NF-kB: the good and the evil in chronic kidney disease? Biochimie 94:2461–2466
Pick E, Keisari Y (1980) A simple colorimetric method for the measurment of hydrogen peroxide produced by cells in culture. J Immunol Methods 38:161–170
Rizvi F, Shukla S, Kakkar P (2014) Essential role of PH damain and leucine-rich repeat protein phosphatase 2 in Nrf2 suppression via modulation of Akt/GSK3β/Fyn kinase axis during oxidative hepatocellular toxicity. Cell Death Dis 5:e1153. doi:10.1038/cddis.2014.118
Ruiz C, Alegría A, Barberá R, Farré R, Lagarda MJ (1999) Lipid peroxidation and antioxidant enzyme activities in patients with type 1 diabetes mellitus. Scand J Clin Lab Invest 59:99–105
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 24:1909–1911
Shen X, Zheng S, Metreveli NS, Epstein PN (2006) Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 55:798–805
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Szaleczky E, Prechl J, Fehér J, Somogyi A (1999) Alterations in enzymatic antioxidant defence in diabetes mellitus—a rational approach. Postgrad Med J 75:13–17
Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, Słomka M, Madro A, Celiński K, Wielosz M (2003) Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepato-Biliary-Pancreat Surg 10:309–315
Tamura M, Oschino N, Chance B (1982) Some characteristics of hydrogen and alkylhydroperoxides metabolizing systems in cardiac tissue. J Biochem 92:1019–1031
Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L, Cui T (2011) Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 60:625–633
Villamena AF (2013) Molecular basis of oxidative stress: chemistry, mechanisms, and disease pathogenesis. Wiley, Hoboken
Wolff SP, Jiang ZY, Hunt JV (1991) Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med 10:339–352
Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN (2004) Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53:1336–1343
Yerra VG, Negi G, Sharma SS, Kumar A (2013) Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kB in diabetic neuropathy. Redox Biol 1:394–397
Young M, McNulty P, Taegtmeyer H (2002) Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation 105:1861–1870
Zhang X, Klein AL, Alberle NS, Norby FL, Ren BH, Duan J, Ren J (2003) Cardiac-specific overexpression of catalase rescues ventricular myocytes from ethanol-induced cardiac contractile defect. J Mol Cell Cardiol 35:645–652
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 173020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanović-Matić, S., Bogojević, D., Martinović, V. et al. Catalase inhibition in diabetic rats potentiates DNA damage and apoptotic cell death setting the stage for cardiomyopathy. J Physiol Biochem 70, 947–959 (2014). https://doi.org/10.1007/s13105-014-0363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-014-0363-y