Abstract
Tenofovir (TNF), a nucleotide analogue of adenosine 5′-monophosphate, is the first-line treatment for chronic HIV/hepatitis B coinfection. There is a need to explore highly sensitive, rapid, and cost-effective quality control techniques to detect the drug. Nickel sulphide @ poly(acrylic acid) coated multi-walled carbon nanotubes (NiS@PAA-MWCNT) composite was synthesized using the one-step solvothermal method. The composite was characterized by UV-vis spectroscopy, Fourier transforms infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and energy-dispersive X-ray spectroscopy. Morphological analysis showed polydispersed NiS on the surface of the poly(acrylic acid) coated multi-walled carbon nanotube (MWCNT). Tenofovir was electrochemically detected in human urine by differential pulse voltammetry (DPV) using the NiS@PAA-MWCNT modified glassy carbon electrode. The linear range was found to be from 20 to 80 µM with a limit of detection of 5.83 × 10−12 M and 5.12 × 10−12 M in phosphate buffer and human urine, respectively. The practical applicability of the modified electrode was evaluated in human urine and in the presence of different interfering agents. The electrode demonstrated good reproducibility, selectivity, and sensitivity. Thus, NiS@PAA-MWCNT nanocomposite can be used in electrochemical sensors for quality control purposes.
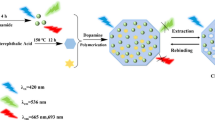
Graphical abstract














Similar content being viewed by others
Availability of Data and Material
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
K. Lacombe, J. Rockstroh, HIV and viral hepatitis coinfections: advances and challenges. Gut. 61, i47–i58 (2012)
M. Simiele, C. Carcieri, A. De Nicolò, A. Ariaudo, M. Sciandra, A. Calcagno, S. Bonora, G. Di Perri, A. D’Avolio, A LC-MS method to quantify tenofovir urinary concentrations in treated patients. J. Pharm. Biomed. Anal. 114, 8–11 (2015)
K. Tsuyuki, H.L. Surratt, M.A. Levi-Minzi, C.L. O’Grady, S.P. Kurtz, The demand for antiretroviral drugs in the illicit marketplace: implications for HIV disease management among vulnerable populations. AIDS. Behav. 19, 857–868 (2015)
O.Y. Rodionova, A.L. Pomerantsev, NIR-based approach to counterfeit-drug detection. Trends. Anal. Chem. 29, 795–803 (2010)
P.Y. Sacré, E. Deconinck, T. De Beer, P. Courselle, R. Vancauwenberghe, P. Chiap, J. Crommen, J.O. De Beer, Comparison and combination of spectroscopic techniques for the detection of counterfeit medicines. J. Pharm. Biomed. Anal. 53, 445–453 (2010)
Y. Roggo, K. Degardin, P. Margot, Identification of pharmaceutical tablets by Raman spectroscopy and chemometrics. Talanta. 81, 988–995 (2010)
S.O. Fakayode, G.A. Baker, D.K. Bwambok et al., Molecular (Raman, NIR, and FTIR) spectroscopy and multivariate analysis in consumable products analysis1. Appl. Spectrosc. Rev. 55, 647–723 (2020)
E. Deconinck, P.Y. Sacré, D. Coomans, J. De Beer, Classification trees based on infrared spectroscopic data to discriminate between genuine and counterfeit medicines. J. Pharm. Biomed. Anal. 57, 68–75 (2012)
R. Urapen, P. Masawat, Novel method for the determination of tetracycline antibiotics in bovine milk based on digital-image-based colorimetry. Int. Dairy. J. 44, 1–5 (2015)
P. Lillsunde, Analytical techniques for drug detection in oral fluid. Ther. Drug. Monit. 30, 181–187 (2008)
R.W. Sparidans, K.M.L. Crommentuyn, J.H.M. Schellens, J.H. Beijnen, Liquid chromatographic assay for the antiviral nucleotide analogue tenofovir in plasma using derivatization with chloroacetaldehyde. J. Chromatogr. B. Anal. Technol. Biomed. Life. Sci. 791, 227–233 (2003)
Biological RK-IJ of P, 2019 U, A review on analytical methods for estimation of tenofovir disoproxil fumarate and emtricitabine in bulk and pharmaceutical (Ijpba.in, 2019)
M. Gandhi, P. Bacchetti, W.C. Rodrigues et al., Development and validation of an immunoassay for tenofovir in urine as a real-time metric of antiretroviral adherence. EClinicalMedicine. 2–3, 22–28 (2018)
H. Kou, X. Du, Y. Li, Q. Fu, Z. Zhu, T. Li, Quantification of tenofovir in human plasma by solid-phase extraction and high-performance liquid chromatography coupled with UV detection. Ther. Drug. Monit. 34, 593–598 (2012)
M. Thevis, D.A. Volmer, Recent instrumental progress in mass spectrometry: advancing resolution, accuracy, and speed of drug detection. Drug. Test. Anal. 4, 242–245 (2012)
O.A. Farghaly, R.S. Abdel Hameed, A.A.H. Abu-Nawwas, Analytical application using modern electrochemical techniques. Int. J. Electrochem. Sci. 9, 3287–3318 (2014)
K. Morawska, T. Popławski, W. Ciesielski, S. Smarzewska, Electrochemical and spectroscopic studies of the interaction of antiviral drug tenofovir with single and double stranded DNA. Bioelectrochemistry. 123, 227–232 (2018)
C.R.R. De, G. Ozcelikay, B. Dogan-topal, S.A. Ozkan, Electrochemical characteristics of tenofovir and its determination in dosage. Rev. Roum. Chim. 62, 569–578 (2017)
L. Shaw, L. Dennany, Applications of electrochemical sensors: forensic drug analysis. Curr. Opin. Electrochem. 3, 23–28 (2017)
Z. Nate, A.A.S. Gill, R. Chauhan, R. Karpoormath, Polyaniline-cobalt oxide nanofibers for simultaneous electrochemical determination of antimalarial drugs: primaquine and proguanil. Microchem. J. 160, 105709 (2021)
V.N. Palakollu, T.E. Chiwunze, A.A.S. Gill, N. Thapliyal, S.M. Maru, R. Karpoormath, Electrochemical sensitive determination of isoprenaline at β-cyclodextrin functionalized graphene oxide and electrochemically generated acid yellow 9 polymer modified electrode. J. Mol. Liq. 248, 953–962 (2017)
Z. Guo, M.V. Reddy, B.M. Goh, A.K.P. San, Q. Bao, K.P. Loh, Electrochemical performance of graphene and copper oxide composites synthesized from a metal-organic framework (Cu-MOF). RSC. Adv. 3, 19051–19056 (2013)
A.A. Ponce, K.J. Klabunde, Chemical and catalytic activity of copper nanoparticles prepared via metal vapor synthesis. J. Mol. Catal. A. Chem. 225, 1–6 (2005)
F.A. Khan, Applications of nanomaterials in human health. Appl. Nanomater. Hum. Heal. (2020). https://doi.org/10.1007/978-981-15-4802-4
M. Arivazhagan, A. Shankar, G. Maduraiveeran, Hollow sphere nickel sulfide nanostructures–based enzyme mimic electrochemical sensor platform for lactic acid in human urine. Microchim. Acta. (2020). https://doi.org/10.1007/s00604-020-04431-3
K. Krishnamoorthy, G.K. Veerasubramani, S.J. Kim, Hydrothermal synthesis, characterization and electrochemical properties of cobalt sulfide nanoparticles. Mater. Sci. Semicond. Process. 40, 781–786 (2015)
S. Kim, S.H. Lee, M. Cho, Y. Lee, Solvent-assisted morphology confinement of a nickel sulfide nanostructure and its application for non-enzymatic glucose sensor. Biosens. Bioelectron. 85, 587–595 (2016)
Y. Wang, J. Zhao, T. Yang, Y. Zhang, D. Tao, Y. Hasebe, Z. Zhang, Electrochemical evaluation of sulfide mineral modified glassy carbon electrode as novel mediated glucose biosensor. J. Electroanal. Chem. 894, 115357 (2021)
C.G. Hu, W.L. Wang, S.X. Wang, W. Zhu, Y. Li, Investigation on electrochemical properties of carbon nanotubes. Diam. Relat. Mater. 12, 1295–1299 (2003)
M.D. Obradović, G.D. Vuković, S.I. Stevanović, V.V. Panić, P.S. Uskoković, A. Kowal, S.L. Gojković, A comparative study of the electrochemical properties of carbon nanotubes and carbon black. J. Electroanal. Chem. 634, 22–30 (2009)
H. Chen, J. Li, D. Shao, X. Ren, X. Wang, Poly(acrylic acid) grafted multiwall carbon nanotubes by plasma techniques for Co(II) removal from aqueous solution. Chem. Eng. J. 210, 475–481 (2012)
J. Theerthagiri, R.A. Senthil, P. Arunachalam, K.A. Bhabu, A. Selvi, J. Madhavan, K. Murugan, A.K. Arof, Electrochemical deposition of carbon materials incorporated nickel sulfide composite as counter electrode for dye-sensitized solar cells. Ionics. 23, 1017–1025 (2017)
A. Singh, A.J. Roberts, R.C.T. Slade, A. Chandra, High electrochemical performance in asymmetric supercapacitors using MWCNT/nickel sulfide composite and graphene nanoplatelets as electrodes. J. Mater. Chem. A. 2, 16723–16730 (2014)
Y. Xiao, J. Wu, J. Lin, G. Yue, J. Lin, M. Huang, Y. Huang, Z. Lan, L. Fan, A high performance Pt-free counter electrode of nickel sulfide/multi-wall carbon nanotube/titanium used in dye-sensitized solar cells. J. Mater. Chem. A. 1, 13885–13889 (2013)
S. Chen, G. Wu, Y. Liu, D. Long, Preparation of poly(acrylic acid) grafted multiwalled carbon nanotubes by a two-step irradiation technique. Macromolecules. 39, 330–334 (2006)
A.A.S. Gill, S. Singh, N. Agrawal, Z. Nate, T.E. Chiwunze, N.B. Thapliyal, R. Chauhan, R. Karpoormath, A poly(acrylic acid)-modified copper-organic framework for electrochemical determination of vancomycin. Microchim. Acta. (2020). https://doi.org/10.1007/s00604-019-4015-3
H.Y. Acar, S. Celebi, N.I. Serttunali, I. Lieberwirth, Development of highly stable and luminescent aqueous CdS quantum dots with the poly(acrylic acid)/mercaptoacetic acid binary coating system. J. Nanosci. Nanotechnol. 9, 2820–2829 (2009)
X. Qi, Z. Wang, S. Ma, L. Wu, S. Yang, J. Xu, Complexation behavior of poly(acrylic acid) and lanthanide ions. Polymer. 55, 1183–1189 (2014)
S. Matsumura, A.R. Hlil, C. Lepiller, J. Gaudet, D. Guay, Z. Shi, S. Holdcroft, A.S. Hay, Stability and utility of pyridyl disulfide functionality in RAFT and conventional radical polymerizations. J. Polym. Sci. A. Polym. Chem. 46, 7207–7224 (2008)
Y. Zhang, X. Liu, Y. Wang, Z. Lou, W. Shan, Y. Xiong, Polyacrylic acid-functionalized graphene oxide for high-performance adsorption of gallium from aqueous solution. J. Colloid. Interface. Sci. 556, 102–110 (2019)
Y. Hu, X. Jiang, Y. Ding, H. Ge, Y. Yuan, C. Yang, Synthesis and characterization of Chitosan-poly(acrylic acid) nanoparticles. Biomaterials. 23, 3193–3201 (2002)
C.S. Thangwane, T. Xaba, M.J. Moloto, The formation of the mixed morphology of nickel sulfide nanoparticles derived from substituted benzimidazole dithiocarbamate nickel (Ii) complexes. Chalcogenide. Lett. 14, 407–417 (2017)
Y. Fazli, S.M. Pourmortazavi, I. Kohsari, M.S. Karimi, M. Tajdari, Synthesis, characterization and photocatalytic property of nickel sulfide nanoparticles. J. Mater. Sci. Mater. Electron. 27, 7192–7199 (2016)
N.A. Batrisya Ismail, F. Abd-Wahab, W.W. Amani Wan Salim, Cyclic voltammetry and electrochemical impedance spectroscopy of partially reduced graphene oxide - PEDOT:PSS transducer for biochemical sensing (2018 IEEE-EMBS Conf. Biomed. Eng. Sci., IEEE, 2018) pp. 330–335
E.P. Randviir, C.E. Banks, Electrochemical impedance spectroscopy: an overview of bioanalytical applications. Anal. Methods. 5, 1098 (2013)
B. Dogan-Topal, B. Bozal-Palabiyik, B. Uslu, S.A. Ozkan, Multi-walled carbon nanotube modified glassy carbon electrode as a voltammetric nanosensor for the sensitive determination of anti-viral drug valganciclovir in pharmaceuticals. Sensors. Actuators. B. Chem. 177, 841–847 (2013)
M. Thiruppathi, N. Thiyagarajan, J.A. Ho, Applications of metals, metal oxides, and metal sulfides in electrochemical sensing and biosensing, in Environmental Chemistry for a Sustainable World. (Springer Nature, Switzerland, 2021), pp. 209–244
R. Chihava, D. Apath, M. Moyo, M. Shumba, V. Chitsa, P. Tshuma, One-pot synthesized nickel-cobalt sulfide-decorated graphene quantum dot composite for simultaneous electrochemical determination of antiretroviral drugs: lamivudine and tenofovir disoproxil fumarate. J. Sensors. 2020, 1–13 (2020)
G. Ozcelikay, B. Dogan-Topal, S.A. Ozkan, An electrochemical sensor based on silver nanoparticles-benzalkonium chloride for the voltammetric determination of antiviral drug tenofovir. Electroanalysis. 30, 943–954 (2018)
N. Festinger, K. Spilarewicz-stanek, K. Borowczyk, D. Guziejewski, Highly sensitive determination of tenofovir in pharmaceutical formulations and patients urine – comparative electroanalytical studies using different sensing methods. Molecules. 27(6), 1992 (2022)
G. Shaw, Bicyclic 5–6 systems: purines. Compr. Heterocycl. Chem. II. A. Rev. Lit. 1982–1995(7), 397–429 (1996)
H. Kamiya, H. Kasai, Formation of 2-hydroxydeoxyadenosine triphosphate, an oxidatively damaged nucleotide, and its incorporation by DNA polymerases. Steady-state kinetics of the incorporation. J. Biol. Chem. 270, 19446–19450 (1995)
Y. Tsurudome, T. Hirano, H. Kamiya, R. Yamaguchi, S. Asami, H. Itoh, H. Kasai, 2-Hydroxyadenine, a mutagenic form of oxidative DNA damage, is not repaired by a glycosylase type mechanism in rat organs. Mutat. Res. DNA. Repair. 408, 121–127 (1998)
H. Kamiya, H. Kasai, 2-Hydroxyadenine (isoguanine) as oxidative DNA damage: its formation and mutation inducibility (Nucleic Acids Symp. Ser., 1995) pp. 233–234
M. Famulok, G. Mayer, M. Blind, Nucleic acid aptamers - from selection in vitro to applications in vivo. Acc. Chem. Res. 33, 591–599 (2000)
S. Tombelli, M. Minunni, M. Mascini, Analytical applications of aptamers. Biosens. Bioelectron. 20, 2424–2434 (2005)
N. Aliakbarinodehi, P. Jolly, N. Bhalla, A. Miodek, G. De Micheli, P. Estrela, S. Carrara, Aptamer-based field-effect biosensor for tenofovir detection. Sci. Rep. 7, 1–10 (2017)
Acknowledgements
College of Health Sciences, University of KwaZulu- Natal (CHS-UKZN). Furthermore, the authors wish to acknowledge Nanotechnology for sustainable development platform of UKZN and UKZN microscopy and microanalysis unit.
Funding
Rajshekhar Karpoormath is funded by the College of Health of Science, UKZN (RM27) and National research Foundation, South Africa (Grant number:129247). Fee Remission was provided by College of Health Science to John Alake.
Author information
Authors and Affiliations
Contributions
John Alake: conceptualization, methodology, investigation, and drafting of manuscript. Zondi Nate: conceptualization, validation, supervision, and review of manuscript. Darko Kwabena Adu: validation. Blessing Wisdom Ike: validation. Ruchika Chauhan: supervision and review of manuscript. Rajshekhar Karpoormath: supervision and funding and resources acquisition.
Corresponding author
Ethics declarations
Ethics Approval
Ethical exemption (00015845) was approved by the Biomedical Research Ethics Committee (BREC), University of KwaZulu-Natal.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Nickel sulphide nanoparticles decorated on poly(acrylic acid) coated multiwalled carbon nanotube was synthesised by a simple one-step solvothermal method.
• The composite modified glassy carbon shows an electro-catalytic behaviour towards tenofovir with a limit of detection of 5.12 × 10−12 M in human urine.
• The electrode demonstrated good reproducibility, selectivity, and sensitivity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alake, J., Nate, Z., Adu, D.K. et al. Facile One-Step Synthesis of Nickel Sulphide Nanoparticles Decorated Poly (Acrylic Acid) Coated Multi-Walled Carbon Nanotube for Detection of Tenofovir in Human Urine. Electrocatalysis 14, 232–246 (2023). https://doi.org/10.1007/s12678-022-00784-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-022-00784-w