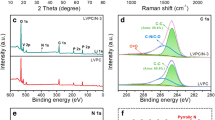
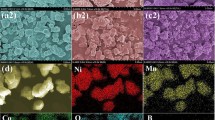
Abstract
The extremely low electrical conductivity and ion-diffusion coefficient of Li2FeSiO4 limits its application as a cathode material in lithium-ion batteries. Therefore, in situ boron-doped Li2FeSi1−xBxO4−δ/C (x = 0, 0.01, 0.03, 0.05 and 0.07) at the Si site was prepared via the solid-state reaction method using pitch as the carbon source. B doping in the lattice structure and a carbon coating on the surface of the composites could effectively enhance the Li+/electron conductivity. Moreover, the reduced particle size of the active material with the relatively high specific area via boron-doped modification could improve the wettability between the electrolyte and cathode. With the synergistic effect of appropriate boron doping and carbon coating, it exhibits a good rate performance, specific capacity, and cycling performance. As a result, the as-prepared Li2FeSi0.95B0.05O4−δ/C cathode showed a high discharge capacity of 160.7 mAh·g−1 at 0.2C, and the capacity retention rate was 96% after 100 cycles at 1.0C. This work presents an effective path for designing advanced cathode materials for lithium-ion batteries.
Graphical abstract

摘要
低的电导率和离子扩散系数限制了Li2FeSiO4 正极材料的实际应用。本文以沥青为碳源,采用固相法构筑了原位硼掺杂Li2FeSi1−xBxO4−δ/C (x = 0, 0.01, 0.03, 0.05 和0.07)复合材料,利用B掺杂协同表面碳包覆层提升材料离子/电子电导率,同时硼掺杂有利于材料获得较高比表面积和较小粒径,增加了材料与电解液的润湿性。在适当的硼掺杂和碳包覆协同作用下,材料表现出优异的倍率性能、比容量和循环性能。结果表明,制备的Li2FeSi0.95B0.05O4−δ/C材料在0.2C下放电容量为160.7 mAh·g-1, 1.0C下循环100次后容量保持率为96%。本研究为设计高性能锂离子电池正极材料提供了一种有效的途径。








Similar content being viewed by others
References
Bruce PG, Scrosati B, Tarascon JM. Nanomaterials for rechargeable lithium batteries. Angew Chem Int Ed. 2010;47(16):2930. https://doi.org/10.1002/anie.200702505.
Zou Y, Chang G, Chen S, Liu T, Xia Y, Chen C, Yang D. Alginate/r-GO assisted synthesis of ultrathin LiFePO4 nanosheets with oriented (0 1 0) facet and ultralow antisite defect. Chem Eng J. 2018;351:340. https://doi.org/10.1016/j.cej.2018.06.104.
Hou LJ, Liu RC, Yuan HY, Kong DZ, Shen WX, Zang JH, Guo J, Dai SG, Wang ML, Xu TT, Li XJ, Wang Y. Micro-structured lepidocrocite-type H1.07Ti1.73O4 as anode for lithium-ion batteries with an ultrahigh rate and long-term cycling performance. Rare Met. 2021;40(6):1391. https://doi.org/10.1007/s12598-020-01618-8.
Qin P, Zhang SQ, Yung KKL, Huang ZF, Gao B. Disclosure of charge storage mechanisms in molybdenum oxide nanobelts with enhanced supercapacitive performance induced by oxygen deficiency. Rare Met. 2021;40(9):2447. https://doi.org/10.1007/s12598-021-01722-3.
Jiang YS, Chen SC, Xiao FM. Electrochemical properties of IrO2-SnO2 coated titanium electrodes in solution containing manganese ion. Chin J Rare Met. 2020,44(10):1063. https://doi.org/10.13373/j.cnki.cjrm.xy19040012.
Long R, Wang GL, Hu ZL, Sun PF, Zhang L. Gradually activated lithium uptake in sodium citrate toward high-capacity organic anode for lithium-ion batteries. Rare Met. 2021;40(6):1366. https://doi.org/10.1007/s12598-020-01502-5.
Liu WJ, Sun XZ, Zhang X, Li C, Wang K, Wen W, Ma YW. Structural evolution of mesoporous graphene/LiNi1/3Co1/3Mn1/3O2 composite cathode for Li-ion battery. Rare Met. 2021;40(3):521. https://doi.org/10.1007/s12598-020-01406-4.
Zhang ZC, Wang X, Lv YC, Mao QZ, Qian ZT. LiNi0.83Co0.12Mn0.05O2 of high nickel ternary cathode material with different precursor preparation processes. Chin J Rare Met. 2021;45(4):410. https://doi.org/10.13373/j.cnki.cjrm.XY20110003.
Sun RX, Yue Y, Cheng XF, Zhang K, Jin SY, Liu GY, Fan YX, Bao Y, Liu XD. Ionic liquid-induced ultrathin and uniform N-doped carbon-wrapped T-Nb2O5 microsphere anode for high-performance lithium-ion battery. Rare Met. 2021;40(11):3205. https://doi.org/10.1007/s12598-020-01681-1.
Yi TF, Qiu LY, Mei J, Qi SY, Cui P, Luo S, Zhu YR, Xie Y, He YB. Porous spherical NiO@NiMoO4@PPy nanoarchitectures as advanced electrochemical pseudocapacitor materials. Sci Bull. 2020;65(7):546. https://doi.org/10.1016/j.scib.2020.01.011.
Li WW, Zhang XJ, Si JJ, Yang J, Sun XY. TiO2-coated LiNi0.9Co0.08Al0.02O2 cathode materials with enhanced cycle performance for Li-ion batteries. Rare Met. 2021;40(7):1719. https://doi.org/10.1007/s12598-020-01483-5.
Ni J, Jiang Y, Bi X, Li L, Lu J. Lithium iron orthosilicate cathode: progress and perspectives. ACS Energy Lett. 2017;2(8):1771. https://doi.org/10.1021/acsenergylett.7b00452.
Nytén A, Abouimrane A, Armand M, Gustafsson T, Thomas JO. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material. Electrochem Commun. 2005;7(2):156. https://doi.org/10.1016/j.elecom.2004.11.008.
Deng LZ, Wu F, Gao XG, Wu WP. Development of a LiFePO4-based high power lithium secondary battery for HEVs applications. Rare Met. 2020;39(12):1457. https://doi.org/10.1007/s12598-014-0316-1.
Larsson P, Ahuja R, Nyten A, Thomas J. An ab initio study of the Li-ion battery cathode material Li2FeSiO4. Electrochem Commun. 2006;8(5):797. https://doi.org/10.1016/j.elecom.2006.03.012.
Peng JM, Chen ZQ, Hu SJ. Conducting network interface modulated rate performance in LiFePO4/C cathode materials. Rare Met. 2022;41(3):951. https://doi.org/10.1007/s12598-021-01838-6.
Deng C, Zhang S, Yang SY, Fu BL, Ma L. Synthesis and characterization of Li2Fe0.97M0.03SiO4 (M=Zn2+, Cu2+, Ni2+) cathode materials for lithium ion batteries. J Power Sources. 2011;196(1):386. https://doi.org/10.1016/j.jpowsour.2010.06.064.
Zhao Y, Wu C, Li J, Guan L. Long cycling life of Li2MnSiO4 lithium battery cathodes under the double protection from carbon coating and graphene network. J Mater Chem A. 2013;1(12):3856. https://doi.org/10.1039/c3ta01521a.
Dominko R. Li2MSiO4 (M=Fe and/or Mn) cathode materials. J Power Sources. 2008;184(2):462. https://doi.org/10.1016/j.jpowsour.2008.02.089.
Ji YR, Weng ST, Li XY. Atomic-scale structural evolution of electrode materials in Li-ion batteries: a review. Rare Met. 2020;39(3):205. https://doi.org/10.1007/s12598-020-01369-6.
Zhang S, Deng C, Fu BL, Yang SY, Ma L. Doping effects of magnesium on the electrochemical performance of Li2FeSiO4 for lithium ion batteries. J Electroanal Chem. 2010;644(2):150. https://doi.org/10.1016/j.jelechem.2009.11.035.
Pushnitsa K, Novikov P, Popovich A, Wang Q. Synthesis of Li2FeSiO4/C composite cathode material for Li-ion batteries and influence of dispersion effect on electrochemical characteristics. Mater Today: Proc. 2020;30:773. https://doi.org/10.1016/j.matpr.2020.01.565.
Li SZ, Li C, Fan YL, Xu JQ, Wang T, Yang ST. Electrochemical performance of LiFePO4 cathode material for Li-ion battery. Rare Met. 2006;25(6):62. https://doi.org/10.1016/S1001-0521(07)60046-1.
Liu L, Wang P, Li J, Shi G, Ma L, Zhao J, An H. Hydrothermal preparation and intrinsic transport properties of nanoscale Li2FeSiO4. Solid State Ionics. 2018;320:353. https://doi.org/10.1016/j.ssi.2018.03.025.
Fujita Y, Iwase H, Shida K, Liao J, Fukui T, Matsuda M. Synthesis of high-performance Li2FeSiO4/C composite powder by spray-freezing/freeze-drying a solution with two carbon sources. J Power Sources. 2017;361:115. https://doi.org/10.1016/j.jpowsour.2017.06.077.
Wang JH, Wang Y, Guo YZ, Liu CW, Dan LL. Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met. 2021;40(1):78. https://doi.org/10.1007/s12598-014-0247-x.
Du X, Zhao H, Lu Y, Gao C, Xia Q, Zhang Z. Electrochemical properties of nanostructured Li2FeSiO4/C synthesized by a simple co-precipitation method. Electrochim Acta. 2016;188:744. https://doi.org/10.1016/j.electacta.2015.12.039.
Sasaki H, Nemoto A, Moriya M, Miyahara M, Hokazono M, Katayama S, Akimoto Y, Nakajima A, Hirano SI. Destruction behavior of carbon hybridized Li2MnSiO4 and Li2FeSiO4 nanoparticles for cathode materials. Ceram Int. 2015;41:S680. https://doi.org/10.1016/j.ceramint.2015.03.139.
Kumar A, Jayakumar OD, Naik VM, Nazri GA, Naik R. Improved electrochemical properties of solvothermally synthesized Li2FeSiO4/C nanocomposites: a comparison between solvothermal and sol-gel methods. Solid State Ionics. 2016;294:15. https://doi.org/10.1016/j.ssi.2016.06.014.
Liu L, Shi G, Lu Y, Lian T, Zhao J, An H, Ma L. Hydrothermal growth kinetics and electrochemical properties of Li2FeSiO4 nanoparticles. J Alloy Compd. 2019;797:1232. https://doi.org/10.1016/j.jallcom.2019.05.072.
Yi TF, Shi L, Han X, Wang F, Zhu Y, Xie Y. Approaching high-performance lithium storage materials by constructing hierarchical CoNiO2@CeO2 nanosheets. Energy Environ Mater. 2020;4(4):586. https://doi.org/10.1002/EEM2.12140.
Vajeeston P. Ionic conductivity enhancement by particle size reduction in Li2FeSiO4. Mater Lett. 2018;218:313. https://doi.org/10.1016/j.matlet.2018.02.041.
Wiriya N, Chantrasuwan P, Kaewmala S, Nash J, Srilomsak S, Meethong N, Limphirat W. Doping effect of manganese on the structural and electrochemical properties of Li2FeSiO4 cathode materials for rechargeable Li-ion batteries. Radiat Phys Chem. 2020;171: 108753. https://doi.org/10.1016/j.radphyschem.2020.108753.
Li L, Han E, Qiao S, Liu H, Shi Y, Yuan W. Synthesis characterization and improved electrochemical performance of Li2FeSiO4/C as cathode for lithium-ion battery by metal doping. Prog Nat Sci: Mater Int. 2019;29(2):111. https://doi.org/10.1016/j.electacta.2016.11.111.
Gao H, Wu Q, Guo M, Yang S, Zhao Y, Kwon YU. Rationally fabricating nitrogen-doped carbon coated nanocrystalline Li2FeSiO4@N-C with excellent Li-ion battery performances. Electrochim Acta. 2019;318:720. https://doi.org/10.1016/j.electacta.2019.06.078.
Liang L, Sun X, Zhang J, Hou L, Sun J, Liu Y, Wang S, Yuan C. In situ synthesis of hierarchical core double-shell Ti-doped LiMnPO4@NaTi2(PO4)3@C/3D graphene cathode with high-rate capability and long cycle life for lithium-ion batteries. Adv Energy Mater. 2019;9(11):1802847. https://doi.org/10.1002/aenm.201802847.
Liang L, Li X, Zhao F, Zhang J, Liu Y, Hou L, Yuan C. Construction and operating mechanism of high-rate Mo-doped Na3V2(PO4)3@C nanowires toward practicable wide-temperature-tolerance Na-ion and hybrid Li/Na-ion batteries. Adv Energy Mater. 2021;11(21):2100287. https://doi.org/10.1002/aenm.202100287.
Li L, Han E, Mi C, Zhu L, Dou L, Shi Y. The effect of Ni or Pb substitution on the electrochemical performance of Li2FeSiO4/C cathode materials. Solid State Ionics. 2019;330:24. https://doi.org/10.1016/j.ssi.2018.12.001.
Sivaraj P, Abhilash KP, Christopher Selvin P, Nalini B. Investigations on the effect of Sm3+ doping on the electrochemical performance of the Li2FeSiO4/C nanocomposite cathode material for lithium ion batteries. Mater Today: Proc. 2019;8:346. https://doi.org/10.1016/j.matpr.2019.02.121.
Gong Z, Yang Y. Recent advances in the research of polyanion-type cathode materials for Li-ion batteries. Energy Environ Sci. 2011;4(9):3223. https://doi.org/10.1039/c0ee00713g.
Li Y, Wang L, Liang F, Yao Y, Zhang K. Enhancing high rate performance and cyclability of LiFePO4 cathode materials for lithium ion batteries by boron doping. J Alloy Compd. 2021;880(6): 160560. https://doi.org/10.1016/j.jallcom.2021.160560.
Sukkabot W. Insight into C, Ge and Sn substitution on structural and electronic properties of Li2FeSiO4: spin density functional theory. Mater Chem Phys. 2019;229:467. https://doi.org/10.1016/j.matchemphys.2019.03.026.
Trócoli R, Franger S, Cruz M, Morales J, Santos-Peña J. Improving the electrochemical properties of nanosized LiFePO4-based electrode by boron doping. Electrochim Acta. 2014;135:558. https://doi.org/10.1016/j.electacta.2014.04.187.
Dahbi M, Urbonaite S, Gustafsson T. Combustion synthesis and electrochemical performance of Li2FeSiO4/C cathode material for lithium-ion batteries. J Power Sources. 2012;205:456. https://doi.org/10.1016/j.jpowsour.2012.01.079.
Pachfule P, Shinde D, Majumder M, Xu Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework. Nat Chem. 2016;8(7):718. https://doi.org/10.1038/NCHEM.2515.
Zhang Z, Liu X, Wang L, Wu Y, Zhao H, Chen B, Xiong W. Fabrication and characterization of carbon-coated Li2FeSiO4 nanoparticles reinforced by carbon nanotubes as high performance cathode materials for lithium-ion batteries. Electrochim Acta. 2015;168:8. https://doi.org/10.1016/j.electacta.2015.04.002.
Jo G, Shanmugam S. Single-step synthetic approach for boron-doped carbons as a non-precious catalyst for oxygen reduction in alkaline medium. Electrochem Commun. 2012;25:101. https://doi.org/10.1016/j.elecom.2012.09.025.
Zhao Y, Yang L, Chen S, Wang X, Ma Y, Wu Q, Jiang Y, Qian W, Hu Z. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? J Am Chem Soc. 2013;135(4):1201. https://doi.org/10.1021/ja310566z.
He H, Sun D, Zhang Q, Fu F, Tang Y, Guo J, Shao M, Wang H. Iron-doped cauliflower-like rutile TiO2 with superior sodium storage properties. ACS Appl Mater Interfaces. 2017;9(7):6093. https://doi.org/10.1021/acsami.6b15516.
Ye K, Li K, Lu Y, Guo Z, Ni N, Liu H, Huang Y, Ji H, Wang P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC, Trends Anal Chem. 2019;116:102. https://doi.org/10.1016/j.trac.2019.05.002.
Gan Q, He H, Zhao K, He Z, Liu S, Yang S. Plasma-induced oxygen vacancies in urchin-like anatase titania coated by carbon for excellent sodium-ion battery anodes. ACS Appl Mater Interfaces. 2018;10(8):7031. https://doi.org/10.1021/acsami.7b13760.
Yang L, Jiang S, Zhao Y, Zhu L, Chen S, Wang X, Wu Q, Ma J, Ma Y, Hu Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew Chem Int Ed. 2011;50(31):7132. https://doi.org/10.1002/anie.201101287.
Yao Y, Jiang Y, Yang H, Sun X, Yu Y. Na3V2(PO4)3 coated by N-doped carbon from ionic liquid as cathode materials for high rate and long-life Na-ion batteries. Nanoscale. 2017;9(30):10880. https://doi.org/10.1039/C7NR03342G.
Ensling D, Stjerndahl M, Nytén A, Gustafsson T, Thomas JO. A comparative XPS surface study of Li2FeSiO4/C cycled with LiTFSI- and LiPF6-based electrolytes. J Mater Chem. 2009;19(1):82. https://doi.org/10.1039/b813099j.
Yang J, Kang X, Hu L, Gong X, Mu S. Nanocrystalline-Li2FeSiO4 synthesized by carbon frameworks as an advanced cathode material for Li-ion batteries. J Mater Chem A. 2014;2(19):6870. https://doi.org/10.1039/c3ta15111e.
Yang J, Kang X, He D, Zheng A, Pan M, Mu S. Graphene activated 3D-hierarchical flower-like Li2FeSiO4 for high-performance lithium-ion batteries. J Mater Chem A. 2015;3(32):16567. https://doi.org/10.1039/C5TA03874J.
Dominko R, Arčon I, Kodre A, Hanžel D, Gaberšček M. In-situ XAS study on Li2MnSiO4 and Li2FeSiO4 cathode materials. J Power Sources. 2009;189(1):51. https://doi.org/10.1016/j.jpowsour.2008.11.077.
Yun NJ, Ha HW, Jeong KH, Park HY, Kim K. Synthesis and electrochemical properties of olivine-type LiFePO4/C composite cathode material prepared from a poly(vinyl alcohol)-containing precursor. J Power Sources. 2006;160(2):1361. https://doi.org/10.1016/j.jpowsour.2006.02.097.
Wang K, Huang X, Zhou T, Wang H, Xie H, Ren Y. Boosted electrochemical properties of porous Li2FeSiO4/C based on Fe-MOFs precursor for lithium ion batteries. Vacuum. 2020;171: 108997. https://doi.org/10.1016/j.vacuum.2019.108997.
Xu Y, Shen W, Zhang A, Liu H, Ma Z. Template-free hydrothermal synthesis of Li2FeSiO4 hollow spheres as cathode materials for lithium-ion batteries. J Mater Chem A. 2014;2(32):12982. https://doi.org/10.1039/c4ta02384f.
Saracibar A, Van der Ven A, Arroyo-de Dompablo ME. Crystal structure, energetics, and electrochemistry of Li2FeSiO4 polymorphs from first principles calculations. Chem Mater. 2012;24(3):495. https://doi.org/10.1021/cm202818u.
Nytén A, Kamali S, Häggström L, Gustafsson T, Thomas JO. The lithium extraction/insertion mechanism in Li2FeSiO4. J Mater Chem. 2006;16(23):2266. https://doi.org/10.1039/b601184e.
Sirisopanaporn C, Masquelier C, Bruce PG, Armstrong AR, Dominko R. Dependence of Li2FeSiO4 electrochemistry on structure. J Am Chem Soc. 2011;133(5):1263. https://doi.org/10.1021/ja109695r.
Huang X, Li X, Wang H, Pan Z, Qu M, Yu Z. Synthesis and electrochemical performance of Li2FeSiO4/C as cathode material for lithium batteries. Solid State Ionics. 2010;181(31):1451. https://doi.org/10.1016/j.ssi.2010.08.007.
Wang MX, Wang K, Huang XB, Zhou T, Xie HS, Ren YR. Improved sodium storage properties of Zr-doped Na3V2(PO4)2F3/C as cathode material for sodium ion batteries. Ceram Int. 2020;46(18):28490. https://doi.org/10.1016/j.ceramint.2020.08.006.
Luo SH, Sun Y, Bao S, Li J, Zhang J, Yi TF. Synthesis of Er-doped LiMnPO4/C by a sol-assisted hydrothermal process with superior rate capability. J Electroanal Chem. 2019;832:196. https://doi.org/10.1016/j.jelechem.2018.10.062.
Zheng Z, Wang Y, Zhang A, Zhang T, Cheng F, Tao Z, Chen J. Porous Li2FeSiO4/C nanocomposite as the cathode material of lithium-ion batteries. J Power Sources. 2012;198:229. https://doi.org/10.1016/j.jpowsour.2011.09.066.
Zhang M. Mn and V co-doped Li2FeSiO4 cathode materials with enhanced electrochemical properties. Int J Electrochem Sci. 2019;14:11289. https://doi.org/10.20964/2019.12.42.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 21771062 and 52174391) and the College Students' Innovation and Entrepreneurship Training Program (No. S2021105330745).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, HX., Zhu, JH., Huang, XB. et al. Enhancing lithium-ion and electric conductive Li2FeSiO4 cathode through in situ boron-doping and carbon-coating strategy. Rare Met. 41, 4055–4064 (2022). https://doi.org/10.1007/s12598-022-02077-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02077-z