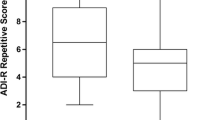
Abstract
Background
The reasons behind the cardinal symptoms of communication deficits and repetitive, stereotyped behaviors that characterize autism spectrum disorder (ASD) remain unknown. The dopamine (DA) system, which regulates motor activity, goal-directed behaviors, and reward function, is believed to play a crucial role in ASD, although the exact mechanism is still unclear. Investigations have shown an association of the dopamine receptor D4 (DRD4) with various neurobehavioral disorders.
Methods
We analyzed the association between ASD and four DRD4 genetic polymorphisms, 5′ flanking 120-bp duplication (rs4646984), rs1800955 in the promoter, exon 1 12 bp duplication (rs4646983), and exon 3 48 bp repeats. We also examined plasma DA and its metabolite levels, DRD4 mRNA expression, and correlations of the studied polymorphisms with these parameters by case–control comparative analyses. The expression of DA transporter (DAT), which is important in regulating the circulating DA level, was also evaluated.
Results
A significantly higher occurrence of rs1800955 “T/TT” was observed in the probands. ASD traits were affected by rs1800955 “T” and the higher repeat alleles of the exon 3 48 bp repeats, rs4646983 and rs4646984. ASD probands exhibited lower DA and norepinephrine levels together with higher homovanillic acid levels than the control subjects. DAT and DRD4 mRNA expression were down-regulated in the probands, especially in the presence of DAT rs3836790 “6R” and rs27072 “CC” and DRD4 rs4646984 higher repeat allele and rs1800955 “T”.
Conclusion
This pioneering investigation revealed a positive correlation between genetic variants, hypodopaminergic state, and impairment in socio-emotional and communication reciprocity in Indian subjects with ASD, warranting further in-depth analysis.




Similar content being viewed by others
Data availability
Data generated for the study are presented in tabular format as Tables, figures, and Additional files. Further details on data will be available from the corresponding author on reasonable request.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2013.
Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ. 2020;69:1–12.
Poovathinal SA, Anitha A, Thomas R, Kaniamattam M, Melempatt N, Anilkumar A, et al. Prevalence of autism spectrum disorders in a semiurban community in south India. Ann Epidemiol. 2016;26:663-5.e8.
Rudra A, Belmonte MK, Soni PK, Banerjee S, Mukerji S, Chakrabarti B. Prevalence of autism spectrum disorder and autistic symptoms in a school-based cohort of children in Kolkata, India. Autism Res. 2017;10:1597–605.
Casanova MF, Frye RE, Gillberg C, Casanova EL. Editorial: co-morbidity and autism spectrum disorder. Front Psychiatry. 2020;11:617395.
Money KM, Stanwood GD. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci. 2013;7:260.
Paval DA. Dopamine hypothesis of autism spectrum disorder. Dev Neurosci. 2017;39:355–60.
Hosenbocus S, Chahal R. A review of executive function deficits and pharmacological management in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2012;21:223–9.
Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74.
Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, et al. Frontostriatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex. 2012;48:183–93.
Fuccillo MV. Striatal circuits as a common node for autism pathophysiology. Front Neurosci. 2016;10:27.
Kosillo P, Bateup HS. Dopaminergic dysregulation in syndromic autism spectrum disorders: insights from genetic mouse models. Front Neural Circuits. 2021;15:700968.
Nguyen M, Roth A, Kyzar EJ, Poudel MK, Wong K, Stewart AM, et al. Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD). Neurochem Int. 2014;66:15–26.
DiCarlo GE, Aguilar JI, Matthies HJ, Harrison FE, Bundschuh KE, West A, et al. Autism-linked dopamine transporter mutation alters striatal dopamine neurotransmission and dopamine-dependent behaviors. J Clin Invest. 2019;129:3407–19.
Chao OY, Pathak SS, Zhang H, Dunaway N, Li JS, Mattern C, et al. Altered dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism. Mol Brain. 2020;13:111.
Kaluzna-Czaplinska J, Socha E, Rynkowski J. Determination of homovanillic acid and vanillylmandelic acid in urine of autistic children by gas chromatography/mass spectrometry. Med Sci Monit. 2010;16:CR445-50.
Martineau J, Barthélémy C, Jouve J, Muh JP, Lelord G. Monoamines (serotonin and catecholamines) and their derivatives in infantile autism: age-related changes and drug effects. Dev Med Child Neurol. 1992;34:593–603.
Alsayouf HA, Talo H, Biddappa ML, Qasaymeh M, Qasem S, De Los RE. Pharmacological intervention in children with autism spectrum disorder with standard supportive therapies significantly improves core signs and symptoms: a single-center, retrospective case series. Neuropsychiatr Dis Treat. 2020;16:2779–94.
Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–11.
Hirsch LE, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2016;2016:CD009043.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12.
Wu J, Xiao H, Sun H, Zou L, Zhu LQ. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012;45:605–20.
Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29.
Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–10.
Klein M, van Donkelaar M, Verhoef E, Franke B. Imaging genetics in neurodevelopmental psychopathology. Am J Med Genet B Neuropsychiatr Genet. 2017;174:485–537.
Wehmuth M, Antoniuk A, Silva KB, Raskinb S, Christoff A, Frigeri HR. Dopamine DRD4 gene polymorphism as a risk factor for epilepsy in autism spectrum disorder. J Biol Med. 2020;4:012–7.
Gadow KD, Devincent CJ, Olvet DM, Pisarevskaya V, Hatchwell E. Association of DRD4 polymorphism with severity of oppositional defiant disorder, separation anxiety disorder and repetitive behaviors in children with autism spectrum disorder. Eur J Neurosci. 2010;32:1058–65.
Reiersen AM, Todorov AA. Association between DRD4 genotype and autistic symptoms in DSM-IV ADHD. J Can Acad Child Adolesc Psychiatr. 2011;20:15–21.
Maitra S, Chatterjee M, Sinha S, Mukhopadhyay K. Dopaminergic gene analysis indicates influence of inattention but not IQ in executive dysfunction of Indian ADHD probands. J Neurogenet. 2019;33:209–17.
Xu FL, Wu X, Zhang JJ, Wang BJ, Yao J. A meta-analysis of data associating DRD4 gene polymorphisms with schizophrenia. Neuropsychiatr Dis Treat. 2018;14:153–64.
Taurines R, Grünblatt E, Schecklmann M, Schwenck C, Albantakis L, Reefschläger L, et al. Altered mRNA expression of monoaminergic candidate genes in the blood of children with attention deficit hyperactivity disorder and autism spectrum disorder. World J Biol Psychiatr. 2011;12:104–8.
Saha S, Chatterjee M, Shom S, Sinha S, Mukhopadhyay K. Functional SLC6A3 polymorphisms differentially affect autism spectrum disorder severity: a study on Indian subjects. Metab Brain Dis. 2022;37:397–410.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
Schopler E, Van Bourgondien ME, Wellman GJ, Love SR. Childhood autism rating scale. 2nd ed. Los Angeles: Western Psychological Services; 2010.
D’Souza UM, Russ C, Tahir E, Mill J, McGuffin P, Asherson PJ, et al. Functional effects of a tandem duplication polymorphism in the 5′ flanking region of the DRD4 gene. Biol Psychiatr. 2004;56:691–7.
Okuyama Y, Ishiguro H, Toru M, Arinami T. A genetic polymorphism in the promoter region of DRD4 associated with expression and schizophrenia. Biochem Biophys Res Commun. 1999;258:292–5.
Catalano M, Nobile M, Novelli E, Nothen MM, Smeraldi E. Distribution of a novel mutation in the first exon of the human dopamine D4 receptor gene in psychotic patients. Biol Psychol. 1993;34:459–64.
Schoots O, Van Tol HH. The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics J. 2003;3:343–8.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Maitra S, Sarkar K, Ghosh P, Karmakar A, Bhattacharjee A, Sinha S, et al. Potential contribution of dopaminergic gene variants in ADHD core traits and comorbidity: a study on eastern Indian probands. Cell Mol Neurobiol. 2014;34:549–64.
Bhaduri N, Das M, Sinha S, Chattopadhyay A, Gangopadhyay PK, Chaudhuri K, et al. Association of dopamine D4 receptor (DRD4) polymorphisms with attention deficit hyperactivity disorder in Indian population. Am J Med Genet Neuropsychiatr Genet. 2006;141:61–6.
Das M, Das Bhowmik A, Bhaduri N, Sarkar K, Ghosh P, Sinha S, et al. Role of gene-gene/gene-environment interaction in the etiology of eastern Indian ADHD probands. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:577–87.
Xu X, Mill J, Sun B, Chen CK, Huang YS, Wu YY, et al. Association study of promoter polymorphisms at the dopamine transporter gene in attention deficit hyperactivity disorder. BMC Psychiatr. 2009;9:3.
Dudbridge F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered. 2008;66:87–98.
Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–82.
Abrahams S, McFie S, Lacerda M, Patricios J, Suter J, September AV, et al. Unravelling the interaction between the DRD2 and DRD4 genes, personality traits and concussion risk. BMJ Open Sport Exerc Med. 2019;5:e000465.
Barkley RA, Smith KM, Fischer M, Navia B. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40bp VNTR) in hyperactive and normal children followed to adulthood. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:487–98.
Kamal M, Elnady G, Abushady A, Khalil M. Association of dopamine D4 receptor gene variants with autism. Int J Res Med Sci. 2015;3:2658–63.
Emanuele E, Boso M, Cassola F, Broglia D, Bonoldi I, Mancini L, et al. Increased dopamine DRD4 receptor mRNA expression in lymphocytes of musicians and autistic individuals: bridging the music-autism connection. Neuro Endocrinol Lett. 2010;31:122–5.
Taurines R, Grünblatt E, Schecklmann M, Schwenck C, Albantakis L, Reefschläger L, et al. Altered mRNA expression of monoaminergic candidate genes in the blood of children with attention deficit hyperactivity disorder and autism spectrum disorder. World J Biol Psychiatr. 2011;12(Suppl 1):104–8.
Schalbroeck R, van Velden FHP, de Geus-Oei LF, Yaqub M, van Amelsvoort T, Booij J, et al. Striatal dopamine synthesis capacity in autism spectrum disorder and its relation with social defeat: an [18F]-FDOPA PET/CT study. Transl Psychiatry. 2021;11:47.
Pascucci T, Colamartino M, Fiori E, Sacco R, Coviello A, Ventura R, et al. P-cresol alters brain dopamine metabolism and exacerbates autism-like behaviors in the BTBR mouse. Brain Sci. 2020;10:233.
Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3:53–67.
Ernst M, Zametkin AJ, Matochik JA, Pascualvaca Cohen RM. Low medial prefrontal dopaminergic activity in autistic children. Lancet. 1997;350:638.
Acknowledgements
The authors are thankful to the study participants for volunteering in the study. In addition, we are grateful to Dr. C Saha for his help during the statistical analysis.
Funding
This work was partially supported by the CSR fund (2021–2022) received from the Ganapati Sugar Industries Ltd, India. No other ad hoc financial support was received.
Author information
Authors and Affiliations
Contributions
SSh: resource, data curation, investigation, formal analysis, writing–original draft. CM: investigation, formal analysis. DN: investigation. SSw: investigation. MK: conceptualization, investigation, writing—review and editing. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
All the procedures involving human participants were performed following the ethical standards of the institutional research committee, which follows the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol was approved by the Human Ethical Committee of the institute (PR-005-19). Written informed consent to participate in the study has been obtained from the participants (or their parent or legal guardian in the case of children under 16).
Consent for publication
Written informed consent was obtained from the participants/parents/caregivers for the anonymous publication of the study findings.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saha, S., Chatterjee, M., Dutta, N. et al. Analysis of neurotransmitters validates the importance of the dopaminergic system in autism spectrum disorder. World J Pediatr 19, 770–781 (2023). https://doi.org/10.1007/s12519-023-00702-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-023-00702-0