Abstract
Introduction
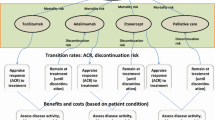
To estimate the cost-effectiveness of tofacitinib for patients with moderate-to-severe rheumatoid arthritis (RA) who failed conventional synthetic disease-modifying antirheumatic drugs from the Chinese healthcare system perspective.
Methods
An individual patient simulation model was used to estimate the lifetime cost and effectiveness. The comparator sequence commenced with etanercept, followed by rituximab-tocilizumab- non-biologic therapy. The intervention sequences were assumed to add tofacitinib to different positions in the comparator sequence. Quality-of-life estimates were generated by mapping Health Assessment Questionnaire scores to utility with the algorithm derived from a Chinese population. Scenario analyses, univariable and probabilistic sensitivity analyses were performed to evaluate the model uncertainty.
Results
Compared with the comparator sequence, patients receiving tofacitinib as the first-, second-, third- and fourth-line treatment gained additional 0.49, 0.59, 0.44 and 0.53 QALYs, respectively, and the use of tofacitinib as the first- and second-line treatment was less costly, whereas the use of tofacitinib as the third- and fourth-line treatment cost an additional $234,998 and $381,116, respectively. This produced an incremental cost-effectiveness ratio of $333.73 and $9669.34/QALY, respectively.
Conclusion
Tofacitinib is estimated to be dominant in both the first- and second-line settings and to be highly cost-effective in both the third- and fourth-line settings.


Similar content being viewed by others
References
Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390(10093):457–68. https://doi.org/10.1016/S0140-6736(17)31618-5.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. https://doi.org/10.1016/S0140-6736(16)30173-8.
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22. https://doi.org/10.1136/annrheumdis-2013-204627.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. https://doi.org/10.1016/S0140-6736(10)60826-4.
Li R, Sun J, Ren LM, Wang HY, Liu WH, Zhang XW, et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology (Oxford). 2012;51(4):721–9. https://doi.org/10.1093/rheumatology/ker370.
Documentation of the Second China National Samples Survey on Disability. China Statistics Press; 2007. http://www.stats.gov.cn/tjsj/ndsj/shehui/2006/html/fu3.htm. Accessed 10 Dec 2020.
Lau CS, Chia F, Harrison A, Hsieh TY, Jain R, Jung SM, et al. APLAR rheumatoid arthritis treatment recommendations. Int J Rheum Dis. 2015;18(7):685–713. https://doi.org/10.1111/1756-185X.12754.
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. https://doi.org/10.1002/art.39480.
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99. https://doi.org/10.1136/annrheumdis-2019-216655.
Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381(9865):451–60. https://doi.org/10.1016/S0140-6736(12)61424-X.
Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159(4):253–61. https://doi.org/10.7326/0003-4819-159-4-201308200-00006.
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86. https://doi.org/10.1056/NEJMoa1310476.
McInnes IB, Kim HY, Lee SH, Mandel D, Song YW, Connell CA, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis. 2014;73(1):124–31. https://doi.org/10.1136/annrheumdis-2012-202442.
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–70. https://doi.org/10.1002/art.37816.
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–19. https://doi.org/10.1056/NEJMoa1112072.
Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K, et al. Safety and efficacy of tofacitinib for up to 95 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21(1):89. https://doi.org/10.1186/s13075-019-1866-2.
Li ZG, Liu Y, Xu HJ, Chen ZW, Bao CD, Gu JR, et al. Efficacy and safety of tofacitinib in chinese patients with rheumatoid arthritis. Chin Med J (Engl). 2018;131(22):2683–92. https://doi.org/10.4103/0366-6999.245157.
Incerti D, Curtis JR, Shafrin J, Lakdawalla DN, Jansen JP. A flexible open-source decision model for value assessment of biologic treatment for rheumatoid arthritis. Pharmacoeconomics. 2019;37(6):829–43. https://doi.org/10.1007/s40273-018-00765-2.
Patton T, Hu H, Luan L, Yang K, Li SC. Mapping between HAQ-DI and EQ-5D-5L in a Chinese patient population. Qual Life Res. 2018;27(11):2815–22. https://doi.org/10.1007/s11136-018-1925-1.
2015 report on Chinese nutrition and chronic disease. http://www.gov.cn/xinwen/2015-06/30/content_2887030.htm. Accessed 10 Dec 2020.
Tian L, Xiong X, Guo Q, Chen Y, Wang L, Dong P, et al. Cost-effectiveness of tofacitinib for patients with moderate-to-severe rheumatoid arthritis in China. Pharmacoeconomics. 2020. https://doi.org/10.1007/s40273-020-00961-z.
An Y, Liu T, He D, Wu L, Li J, Liu Y, et al. The usage of biological DMARDs and clinical remission of rheumatoid arthritis in China: a real-world large scale study. Clin Rheumatol. 2017;36(1):35–43. https://doi.org/10.1007/s10067-016-3424-5.
Carlson JJ, Ogale S, Dejonckheere F, Sullivan SD. Economic evaluation of tocilizumab monotherapy compared to adalimumab monotherapy in the treatment of severe active rheumatoid arthritis. Value Health. 2015;18(2):173–9. https://doi.org/10.1016/j.jval.2014.10.013.
Brennan A, Bansback N, Reynolds A, Conway P. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology (Oxford). 2004;43(1):62–72. https://doi.org/10.1093/rheumatology/keg451.
Diamantopoulos A, Benucci M, Capri S, Berger W, Wintfeld N, Giuliani G, et al. Economic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in Italy. J Med Econ. 2012;15(3):576–85. https://doi.org/10.3111/13696998.2012.665110.
Madan J, Ades AE, Welton NJ. An overview of models used in economic analyses of biologic therapies for arthritis–from current diversity to future consensus. Rheumatology (Oxford). 2011;50(Suppl 4):10–8. https://doi.org/10.1093/rheumatology/ker240.
Claxton L, Jenks M, Taylor M, Wallenstein G, Mendelsohn AM, Bourret JA, et al. An economic evaluation of tofacitinib treatment in rheumatoid arthritis: modeling the cost of treatment strategies in the United States. J Manag Care Spec Pharm. 2016;22(9):1088–102. https://doi.org/10.18553/jmcp.2016.22.9.1088.
Claxton L, Taylor M, Soonasra A, Bourret JA, Gerber RA. An economic evaluation of tofacitinib treatment in rheumatoid arthritis after methotrexate or after 1 or 2 TNF inhibitors from a U.S. payer perspective. J Manag Care Spec Pharm. 2018;24(10):1010–7. https://doi.org/10.18553/jmcp.2018.17220.
Strand V, Miller P, Williams SA, Saunders K, Grant S, Kremer J. Discontinuation of biologic therapy in rheumatoid arthritis: analysis from the corrona RA registry. Rheumatol Ther. 2017;4(2):489–502. https://doi.org/10.1007/s40744-017-0078-y.
Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610. https://doi.org/10.3310/hta20350.
Singh JA, Wells GA, Christensen R, Tanjong GE, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011. https://doi.org/10.1002/14651858.CD008794.pub2.
Tosh J, Brennan A, Wailoo A, Bansback N. The Sheffield rheumatoid arthritis health economic model. Rheumatology (Oxford). 2011;50(4):26–31. https://doi.org/10.1093/rheumatology/ker243.
Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the Medicare program: a cost-effectiveness analysis. Arthritis Rheum. 2008;58(4):939–46. https://doi.org/10.1002/art.23374.
Wolfe F, Michaud K. The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study. Arthritis Res Ther. 2010;12(2):R35. https://doi.org/10.1186/ar2944.
Michaud K, Wallenstein G, Wolfe F. Treatment and nontreatment predictors of health assessment questionnaire disability progression in rheumatoid arthritis: a longitudinal study of 18,485 patients. Arthritis Care Res (Hoboken). 2011;63(3):366–72. https://doi.org/10.1002/acr.20405.
Life tables for WHO member states. https://apps.who.int/gho/data/?theme=main&vid=60340. 2016. Accessed 10 May 2020.
Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1530–42. https://doi.org/10.1002/art.11024.
Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):54–9. https://doi.org/10.3899/jrheum.110491.
Wu B, Wilson A, Wang FF, Wang SL, Wallace DJ, Weisman MH, et al. Cost effectiveness of different treatment strategies in the treatment of patients with moderate to severe rheumatoid arthritis in china. PLoS ONE. 2012;7(10):e47373. https://doi.org/10.1371/journal.pone.0047373.
Health care and personal articles of consumer price Indices. National Bureau of Statistics of China. http://www.stats.gov.cn/. Accessed 27 Nov 2020
China Statistical Yearbook 2020. http://www.stats.gov.cn/tjsj/ndsj/2020/indexeh.htm. Accessed 11 Dec 2020.
Chen DY, Hsu PN, Tang CH, Claxton L, Valluri S, Gerber RA. Tofacitinib in the treatment of moderate-to-severe rheumatoid arthritis: a cost-effectiveness analysis compared with adalimumab in Taiwan. J Med Econ. 2019;22(8):777–87. https://doi.org/10.1080/13696998.2019.1606813.
Claxton L, Taylor M, Gerber RA, Gruben D, Moynagh D, Singh A, et al. Modelling the cost-effectiveness of tofacitinib for the treatment of rheumatoid arthritis in the United States. Curr Med Res Opin. 2018;34(11):1991–2000. https://doi.org/10.1080/03007995.2018.1497957.
Lee MY, Park SK, Park SY, Byun JH, Lee SM, Ko SK, et al. Cost-effectiveness of tofacitinib in the treatment of moderate to severe rheumatoid arthritis in South Korea. Clin Ther. 2015;37(8):1662–76. https://doi.org/10.1016/j.clinthera.2015.07.001.
Navarro F, Martinez-Sesmero JM, Balsa A, Peral C, Montoro M, Valderrama M, et al. Cost-effectiveness analysis of treatment sequences containing tofacitinib for the treatment of rheumatoid arthritis in Spain. Clin Rheumatol. 2020;39(10):2919–30. https://doi.org/10.1007/s10067-020-05087-3.
Scholz S, Mittendorf T. Modeling rheumatoid arthritis using different techniques - a review of model construction and results. Health Econ Rev. 2014;4(1):18. https://doi.org/10.1186/s13561-014-0018-2.
Jansen JP, Incerti D, Mutebi A, Peneva D, MacEwan JP, Stolshek B, et al. Cost-effectiveness of sequenced treatment of rheumatoid arthritis with targeted immune modulators. J Med Econ. 2017;20(7):703–14. https://doi.org/10.1080/13696998.2017.1307205.
Norman R, Cronin P, Viney R, King M, Street D, Ratcliffe J. International comparisons in valuing EQ-5D health states: a review and analysis. Value Health. 2009;12(8):1194–200. https://doi.org/10.1111/j.1524-4733.2009.00581.x.
Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib up to 95 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020. https://doi.org/10.1136/rmdopen-2020-001395.
Wu B, Song Y, Leng L, Bucala R, Lu LJ. Treatment of moderate rheumatoid arthritis with different strategies in a health resource-limited setting: a cost-effectiveness analysis in the era of biosimilars. Clin Exp Rheumatol. 2015;33(1):20–6.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were supported by the National Natural Science Foundation of China (no. 71874209) and Hunan Provincial Natural Science Foundation of China (no. 2019JJ40411).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Authors’ Contributions
Wan XiaoMin and Chongqing Tan, Sini Li, Lidan Yi and Xiaohui Zeng developed the economic model, performed the analyses, interpreted the results and drafted the article. Liubao Peng, Shuxia Qin and Liting Wang collected and reviewed the data. XiaoMin Wan contributed to the conception and design of the primary model. All authors read and approved the final article.
Disclosures
Chongqing Tan, Sini Li, Lidan Yi, Xiaohui Zeng, Liubao Peng, Shuxia Qin, Liting Wang and Xiaomin Wan declare they have nothing to disclose.
Compliance with Ethics Guidelines
The model used in this analysis was based on previously conducted studies and other economic models; no studies with human participants or animals were performed by any of the authors. Ethics committee approval is not required.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, C., Li, S., Yi, L. et al. Tofacitinib in the Treatment of Moderate-to-Severe Rheumatoid Arthritis in China: A Cost-Effectiveness Analysis Based on a Mapping Algorithm Derived from a Chinese Population. Adv Ther 38, 2571–2585 (2021). https://doi.org/10.1007/s12325-021-01733-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01733-7