Abstract
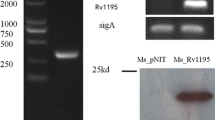
Tuberculosis (TB), a bacterial infectious disease caused by Mycobacterium tuberculosis (M. tuberculosis), is a significant global public health problem. Mycobacterium tuberculosis expresses a unique family of PE_PGRS proteins that have been implicated in pathogenesis. Despite numerous studies, the functions of most PE_PGRS proteins in the pathogenesis of mycobacterium infections remain unclear. PE_PGRS45 (Rv2615c) is only found in pathogenic mycobacteria. In this study, we successfully constructed a recombinant Mycobacterium smegmatis (M. smegmatis) strain which heterologously expresses the PE_PGRS45 protein. We found that overexpression of this cell wall-associated protein enhanced bacterial viability under stress in vitro and cell survival in macrophages. MS_PE_PGRS45 decreased the secretion of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12p40, and TNF-α. We also found that MS_PE_PGRS45 increased the expression of the anti-inflammatory cytokine IL-10 and altered macrophage-mediated immune responses. Furthermore, PE_PGRS45 enhanced the survival rate of M. smegmatis in macrophages by inhibiting cell apoptosis. Collectively, our findings show that PE_PGRS45 is a virulent factor actively involved in the interaction with the host macrophage.






Similar content being viewed by others
Data Availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Abo-Kadoum, M. A., Assad, M., Ali, M. K., Uae, M., Nzaou, S., Gong, Z., Moaaz, A., Lambert, N., Eltoukhy, A., & Xie, J. (2021). Mycobacterium tuberculosis PE17 (Rv1646) promotes host cell apoptosis via host chromatin remodeling mediated by reduced H3K9me3 occupancy. Microbial Pathogenesis,159, 105147.
Ali, M. K., Zhen, G., Nzungize, L., Stojkoska, A., Duan, X., Li, C., Duan, W., Xu, J., & Xie, J. (2020). Mycobacterium tuberculosis PE31 (Rv3477) attenuates host cell apoptosis and promotes recombinant M. smegmatis intracellular survival via up-regulating GTPase guanylate binding protein-1. Frontiers in Cellular and Infection. Microbiology,10, 40.
Bachhawat, N. (2018). PE-only/PE_PGRS proteins of Mycobacterium tuberculosis contain a conserved tetra-peptide sequence DEVS/DXXS that is a potential caspase-3 cleavage motif. Journal of Biosciences,43, 597–604.
Bagcchi, S. (2023). WHO’s Global tuberculosis report 2022. The Lancet Microbe,4, e20.
Basu, S., Pathak, S. K., Banerjee, A., Pathak, S., Bhattacharyya, A., Yang, Z., Talarico, S., Kundu, M., & Basu, J. (2007). Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by toll-like receptor 2-dependent release of Tumor necrosis factor-α. The Journal of Biological Chemistry,282, 1039–1050.
Behar, S. M., Martin, C. J., Booty, M. G., Nishimura, T., Zhao, X., Gan, H. X., Divangahi, M., & Remold, H. G. (2011). Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunology,4, 279–287.
Boom, W. H., Schaible, U. E., & Achkar, J. M. (2021). The knowns and unknowns of latent Mycobacterium tuberculosis Infection. The Journal of Clinical Investigation,131, e136222.
Cadieux, N., Parra, M., Cohen, H., Maric, D., Morris, S. L., & Brennan, M. J. (2011). Induction of cell death after localization to the host cell mitochondria by the Mycobacterium tuberculosis PE_PGRS33 protein. Microbiology,157, 793–804.
Campuzano, J., Aguilar, D., Arriaga, K., León, J. C., Salas-Rangel, L. P., González-y-Merchand, J., Hernández-Pando, R., & Espitia, C. (2007). The PGRS domain of Mycobacterium tuberculosis PE_PGRS Rv1759c antigen is an efficient subunit vaccine to prevent reactivation in a murine model of chronic Tuberculosis. Vaccine,25, 3722–3729.
Chai, Q., Wang, L., Liu, C. H., & Ge, B. (2020). New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cellular & Molecular Immunology,17, 901–913.
Chakaya, J., Petersen, E., Nantanda, R., Mungai, B. N., Migliori, G. B., Amanullah, F., Lungu, P., Ntoumi, F., Kumarasamy, N., Maeurer, M., et al. (2022). The WHO Global Tuberculosis 2021 Report - not so good news and turning the tide back to end TB. International Journal of Infectious Diseases,124, S26–S29.
Cilfone, N. A., Ford, C. B., Marino, S., Mattila, J. T., Gideon, H. P., Flynn, J. L., Kirschner, D. E., & Linderman, J. J. (2015). Computational modeling predicts IL-10 control of lesion sterilization by balancing early host immunity-mediated antimicrobial responses with caseation during Mycobacterium tuberculosis Infection. Journal of Immunology,194, 664–677.
Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., Gordon, S. V., Eiglmeier, K., Gas, S., Barry, C. E., et al. (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature,393, 537–544.
Cooper, A. M., & Khader, S. A. (2008). The role of cytokines in the initiation, expansion, and control of cellular immunity to Tuberculosis. Immunological Reviews,226, 191–204.
Dan, L., Jianping, X., Ruzhen, G., & Honghai, W. (2009). Cloning and characterization of Rv0621 gene related to surfactant stress tolerance in Mycobacterium tuberculosis. Molecular Biology Reports,36, 1811–1817.
Dao, D. N., Kremer, L., Guérardel, Y., Molano, A., Jacobs, W. R., Jr., Porcelli, S. A., & Briken, V. (2004). Mycobacterium tuberculosis Lipomannan induces apoptosis and interleukin-12 production in macrophages. Infection and Immunity,72, 2067–2074.
de Martino, M., Lodi, L., Galli, L., & Chiappini, E. (2019). Immune response to Mycobacterium tuberculosis: A narrative review. Frontiers in Pediatrics,7, 350.
Deng, W., Long, Q., Zeng, J., Li, P., Yang, W., Chen, X., & Xie, J. (2017). Mycobacterium tuberculosis PE_PGRS41 enhances the intracellular survival of M. Smegmatis within macrophages via blocking innate immunity and inhibition of host defense. Scientific Reports,7, 46716.
Dheenadhayalan, V., Delogu, G., Sanguinetti, M., Fadda, G., & Brennan, M. J. (2006). Variable expression patterns of Mycobacterium tuberculosis PE_PGRS genes: Evidence that PE_PGRS16 and PE_PGRS26 are inversely regulated in vivo. Journal of Bacteriology,188, 3721–3725.
Ehrt, S., & Schnappinger, D. (2009). Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cellular Microbiology,11, 1170–1178.
Fairbairn, I. P. (2004). Macrophage apoptosis in host immunity to mycobacterial Infections. Biochemical Society Transactions,32, 496–498.
Feng, L., Hu, J., Zhang, W., Dong, Y., Xiong, S., & Dong, C. (2020). RELL1 inhibits autophagy pathway and regulates Mycobacterium tuberculosis survival in macrophages. Tuberculosis,120, 101900.
Ferguson, J. S., Voelker, D. R., McCormack, F. X., & Schlesinger, L. S. (1999). Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. Journal of Immunology,163, 312–321.
Fratazzi, C., Arbeit, R. D., Carini, C., Balcewicz-Sablinska, M. K., Keane, J., Kornfeld, H., & Remold, H. G. (1999). Macrophage apoptosis in mycobacterial Infections. Journal of Leukocyte Biology,66, 763–764.
Gey van Pittius, N. C., Sampson, S. L., Lee, H., Kim, Y., van Helden, P. D., & Warren, R. M. (2006). Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evolutionary Biology,6, 95.
Gong, Z., Yang, W., Zhang, H., Xiang, X., Zeng, J., Han, S., Yang, J., & Xie, J. (2020). Mycobacterium tuberculosis Rv3717 enhances the survival of Mycolicibacterium smegmatis by inhibiting host innate immune and caspase-dependent apoptosis. Infection Genetics and Evolution,84, 104412.
Hirsch, C. S., Toossi, Z., Othieno, C., Johnson, J. L., Schwander, S. K., Robertson, S., Wallis, R. S., Edmonds, K., Okwera, A., Mugerwa, R., et al. (1999). Depressed T-cell interferon-γ responses in pulmonary Tuberculosis: Analysis of underlying mechanisms and modulation with therapy. The Journal of Infectious Diseases,180, 2069–2073.
Houben, R. M., & Dodd, P. J. (2016). The global burden of latent Tuberculosis Infection: A re-estimation using mathematical modelling. PLoS Medicine,13, e1002152.
Huang, Y., Wang, Y., Bai, Y., Wang, Z. G., Yang, L., & Zhao, D. (2010). Expression of PE_PGRS 62 protein in Mycobacterium smegmatis decrease mRNA expression of proinflammatory cytokines IL-1β, IL-6 in macrophages. Molecular and Cellular Biochemistry,340, 223–229.
Huang, Y., Zhou, X., Bai, Y., Yang, L., Yin, X., Wang, Z., & Zhao, D. (2012). Phagolysosome maturation of macrophages was reduced by PE_PGRS 62 protein expressing in Mycobacterium smegmatis and induced in IFN-γ priming. Veterinary Microbiology,160, 117–125.
Johansson, J., & Curstedt, T. (1997). Molecular structures and interactions of pulmonary surfactant components. European Journal of Biochemistry,244, 675–693.
Jouanguy, E., Döffinger, R., Dupuis, S., Pallier, A., Altare, F., & Casanova, J. L. (1999). IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Current Opinion in Immunology,11, 346–351.
Lakshminarayan, H., Narayanan, S., Bach, H., Sundaram, K. G., & Av-Gay, Y. (2008). Molecular cloning and biochemical characterization of a serine threonine protein kinase, PknL, from Mycobacterium tuberculosis. Protein Expression and Purification,58, 309–317.
Lam, A., Prabhu, R., Gross, C. M., Riesenberg, L. A., Singh, V., & Aggarwal, S. (2017). Role of apoptosis and autophagy in Tuberculosis. American Journal of Physiology Lung Cellular and Molecular Physiology,313, L218–L229.
Li, J., Chai, Q. Y., Zhang, Y., Li, B. X., Wang, J., Qiu, X. B., & Liu, C. H. (2015). Mycobacterium tuberculosis Mce3E suppresses host innate immune responses by targeting ERK1/2 signaling. Journal of Immunology,194, 3756–3767.
Lin, P. L., & Flynn, J. L. (2010). Understanding latent Tuberculosis: A moving target. Journal of Immunology,185, 15–22.
Liu, D., Hao, K., Wang, W., Peng, C., Dai, Y., Jin, R., Xu, W., He, L., Wang, H., & Wang, H. (2017). Rv2629 overexpression delays Mycobacterium smegmatis and Mycobacteria tuberculosis entry into log-phase and increases pathogenicity of Mycobacterium smegmatis in mice. Frontiers in Microbiology,8, 2231.
Long, Q., Xiang, X., Yin, Q., Li, S., Yang, W., Sun, H., Liu, Q., Xie, J., & Deng, W. (2019). PE_PGRS62 promotes the survival of Mycobacterium smegmatis within macrophages via disrupting ER stress-mediated apoptosis. Journal of Cellular Physiology,234, 19774–19784.
Malik, Z. A., Iyer, S. S., & Kusner, D. J. (2001). Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: Contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. Journal of Immunology,166, 3392–3401.
Manganelli, R., Dubnau, E., Tyagi, S., Kramer, F. R., & Smith, I. (1999). Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Molecular Microbiology,31, 715–724.
Medha, Joshi, H., Sharma, S., & Sharma, M. (2023a). Elucidating the function of hypothetical PE_PGRS45 protein of Mycobacterium tuberculosis as an oxido-reductase: A potential target for drug repurposing for the treatment of Tuberculosis. Journal of Biomolecular Structure & Dynamics,41, 10009–10025.
Medha, P., Sharma, S., & Sharma, M. (2023b). PE_PGRS45 (Rv2615c) protein of Mycobacterium tuberculosis perturbs mitochondria of macrophages. Immunology and Cell Biology,101, 829–846.
Muttucumaru, D. G., Smith, D. A., McMinn, E. J., Reese, V., Coler, R. N., & Parish, T. (2011). Mycobacterium tuberculosis Rv0198c, a putative matrix metalloprotease is involved in pathogenicity. Tuberculosis,91, 111–116.
Nagabhushanam, V., Solache, A., Ting, L. M., Escaron, C. J., Zhang, J. Y., & Ernst, J. D. (2003). Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-γ. Journal of Immunology,171, 4750–4757.
Newton, G. L., Koledin, T., Gorovitz, B., Rawat, M., Fahey, R. C., & Av-Gay, Y. (2003). The glycosyltransferase gene encoding the enzyme catalyzing the first step of mycothiol biosynthesis (mshA). Journal of Bacteriology,185, 3476–3479.
Pan, H., Yan, B. S., Rojas, M., Shebzukhov, Y. V., Zhou, H., Kobzik, L., Higgins, D. E., Daly, M. J., Bloom, B. R., & Kramnik, I. (2005). Ipr1 gene mediates innate immunity to Tuberculosis. Nature,434, 767–772.
Rovetta, A. I., Peña, D., Hernández, D., Pino, R. E., Recalde, G. M., Pellegrini, J., Bigi, F., Musella, R. M., Palmero, D. J., Gutierrez, M., Colombo, M. I., et al. (2014). IFNG-mediated immune responses enhance autophagy against Mycobacterium tuberculosis antigens in patients with active Tuberculosis. Autophagy,10, 2109–2121.
Ruan, C., Li, J., Niu, J., Li, P., Huang, Y., Li, X., Duan, W., Yan, S., Zhen, J., & Xie, J. (2020). Mycobacterium tuberculosis Rv0426c promotes recombinant mycobacteria intracellular survival via manipulating host inflammatory cytokines and suppressing cell apoptosis. Infection Genetics and Evolution,77, 104070.
Shah, N. S., Auld, S. C., Brust, J. C., Mathema, B., Ismail, N., Moodley, P., Mlisana, K., Allana, S., Campbell, A., Mthiyane, T., et al. (2017). Transmission of extensively drug-resistant Tuberculosis in South Africa. The New England Journal of Medicine,376, 243–253.
Sharma, T., Grover, S., Arora, N., Ehtesham, P. M., & Hasnain, S. E. (2020). PGRS Domain of Rv0297 of Mycobacterium tuberculosis is involved in modulation of macrophage functions to favor bacterial persistence. Frontiers in Cellular and Infection Microbiology,10, 451.
Singh, P. P., & Goyal, A. (2013). Interleukin-6: a potent biomarker of mycobacterial infection. Springerplus,2, 686.
Singh, V. K., Berry, L., Bernut, A., Singh, S., Carrère-Kremer, S., Viljoen, A., Alibaud, L., Majlessi, L., Brosch, R., Chaturvedi, V., et al. (2016). A unique PE_PGRS protein inhibiting host cell cytosolic defenses and sustaining full virulence of Mycobacterium marinum in multiple hosts. Cellular Microbiology,18, 1489–1507.
Sousa, J., Cá, B., Maceiras, A. R., Simões-Costa, L., Fonseca, K. L., Fernandes, A. I., Ramos, A., Carvalho, T., Barros, L., & Magalhães, C. (2020). Mycobacterium tuberculosis associated with severe tuberculosis evades cytosolic surveillance systems and modulates IL-1β production. Nature Communications,11, 1949.
Spira, A., Carroll, J. D., Liu, G., Aziz, Z., Shah, V., Kornfeld, H., & Keane, J. (2003). Apoptosis genes in human alveolar macrophages infected with virulent or attenuated Mycobacterium tuberculosis: A pivotal role for Tumor necrosis factor. American Journal of Respiratory Cell and Molecular Biology,29, 545–551.
Srivastava, V., Jain, A., Srivastava, B. S., & Srivastava, R. (2008). Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. Tuberculosis,88, 171–177.
Tian, C., & Jian-Ping, X. (2010). Roles of PE_PGRS family in Mycobacterium tuberculosis pathogenesis and novel measures against Tuberculosis. Microbial Pathogenesis,49, 311–314.
Tiwari, B. M., Kannan, N., Vemu, L., & Raghunand, T. R. (2012). The Mycobacterium tuberculosis PE proteins Rv0285 and Rv1386 modulate innate immunity and mediate bacillary survival in macrophages. PLoS One,7, e51686.
Tiwari, B., Soory, A., & Raghunand, T. R. (2014). An immunomodulatory role for the Mycobacterium tuberculosis region of difference 1 locus proteins PE35 (Rv3872) and PPE68 (Rv3873). The FEBS Journal,281, 1556–1570.
Tiwari, B., Ramakrishnan, U. M., & Raghunand, T. R. (2015). The Mycobacterium tuberculosis protein pair PE9 (Rv1088)-PE10 (Rv1089) forms heterodimers and induces macrophage apoptosis through toll-like receptor 4. Cellular Microbiology,17, 1653–1669.
Tsenova, L., Bergtold, A., Freedman, V. H., Young, R. A., & Kaplan, G. (1999). Tumor necrosis factor α is a determinant of pathogenesis and Disease progression in mycobacterial Infection in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America,96, 5657–5662.
Tundup, S., Mohareer, K., & Hasnain, S. E. (2014). Mycobacterium tuberculosis PE25/PPE41 protein complex induces necrosis in macrophages: Role in virulence and Disease reactivation? FEBS Open Bio,4, 822–828.
Valone, S. E., Rich, E. A., Wallis, R. S., & Ellner, J. J. (1988). Expression of Tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infection and Immunity,56, 3313–3315.
Weiss, G., & Schaible, U. E. (2015). Macrophage defense mechanisms against intracellular bacteria. Immunological Reviews,264, 182–203.
Xie, Y., Zhou, Y., Liu, S., & Zhang, X. L. (2021). PE_PGRS: Vital proteins in promoting mycobacterial survival and modulating host immunity and metabolism. Cellular Microbiology,23, e13290.
Xu, S., Shen, L., Liu, H., Tang, Y., & Zhang, L. (2022). Overexpression of LprO protein in Mycobacterium smegmatis promotes apoptosis of macrophages. Progress in Modern Biomedicine,21, 4001–4008. https://doi.org/10.13241/j.cnki.pmb.2022.21.001
Xu, T., Li, M., Wang, C., Yuan, M., Chang, X., Qian, Z., Li, B., Sun, M., & Wang, H. (2021). Codon optimization, soluble expression and purification of PE_PGRS45 gene from Mycobacterium tuberculosis and preparation of its polyclonal antibody protein. Journal of Microbiology and Biotechnology,31, 1583–1590.
Yan, S., Xu, M., Wang, R., Li, Q., Yu, Z., & Xie, J. (2017). Overexpression of Rv2788 increases mycobacterium stresses survival. Microbiological Research,195, 51–59.
Yan, S., Zhen, J., Li, Y., Zhang, C., Stojkoska, A., Lambert, N., Li, Q., Li, P., & Xie, J. (2019). Mce-associated protein Rv0177 alters the cell wall structure of Mycobacterium smegmatis and promotes macrophage apoptosis via regulating the cytokines. International Immunopharmacology,66, 205–214.
Yang, W., Deng, W., Zeng, J., Ren, S., Ali, M. K., Gu, Y., Li, Y., & Xie, J. (2017). Mycobacterium tuberculosis PE_PGRS18 enhances the intracellular survival of M. Smegmatis via altering host macrophage cytokine profiling and attenuating the cell apoptosis. Apoptosis,22, 502–509.
Yang, W., Liu, M., Yu, X., Huang, Y., Zeng, J., Dai, Y., Luo, H., Huang, Q., Fan, L., & Xie, J. (2021). Mycobacterium tuberculosis Rv1515c antigen enhances survival of M. Smegmatis within macrophages by disrupting the host defence. Microbial Pathogenesis,153, 104778.
Young, C., Walzl, G., & Du Plessis, N. (2020). Therapeutic host-directed strategies to improve outcome in Tuberculosis. Mucosal Immunology,13, 190–204.
Zhang, Z. (2007). Ph.D. thesis. Screening and analysis of in vivo induced genes of Mycobacterium tuberculosis. Beijing Tuberculosis and Thoracic Tumor Research Institute.
Acknowledgements
This research was supported by the Anhui Provincial Natural Science Foundation (1908085MH252, 2008085QH405), Anhui Province Key Laboratory of Immunology in Chronic Diseases Open Project (KLICD-2022-Z3), Anhui Province Key Laboratory of Clinical and Preclinical Research in Respiratory Disease Open Project (HX2022Z02), the 512 Talent Cultivation Plan of Bengbu Medical College (by51201309), and the National University Students’ Innovation and Entrepreneurship Training Program (202210367069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, T., Wang, C., Li, M. et al. Mycobacterium tuberculosis PE_PGRS45 (Rv2615c) Promotes Recombinant Mycobacteria Intracellular Survival via Regulation of Innate Immunity, and Inhibition of Cell Apoptosis. J Microbiol. 62, 49–62 (2024). https://doi.org/10.1007/s12275-023-00101-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-023-00101-0