Abstract
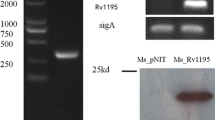
Mycobacterium tuberculosis PE/PPE family proteins, named after the presence of conserved PE (Pro-Glu) and PPE (Pro-Pro-Glu) domains at N-terminal, are prevalent in M. tuberculosis genome. The function of most PE/PPE family proteins remains elusive. To characterize the function of PE_PGRS18, the encoding gene was heterologously expressed in M. smegmatis, a nonpathogenic mycobacterium. The recombinant PE_PGRS18 is cell wall associated. M. smegmatis PE_PGRS18 recombinant showed differential response to stresses and altered the production of host cytokines IL-6, IL-1β, IL-12p40 and IL-10, as well as enhanced survival within macrophages largely via attenuating the apoptosis of macrophages. In summary, the study firstly unveiled the role of PE_PGRS18 in physiology and pathogenesis of mycobacterium.






Similar content being viewed by others
References
Lin PL, Flynn JL (2010) Understanding latent tuberculosis: a moving target. J Immunol 185(1):15–22
Abdallah AM, Verboom T, Weerdenburg EM et al (2009) PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Mol Microbiol 73(3):329–340
Delogu G, Pusceddu C, Bua A et al (2004) Rv1818c-encoded PE_PGRS protein of Mycobacterium tuberculosis is surface exposed and influences bacterial cell structure. Mol Microbiol 52(3):725–733
Bachhawat N, Singh B (2007) Mycobacterial PE PGRS proteins contain calcium-binding motifs with parallel β-roll folds. Genom Proteom Bioinform 5(3–4):236–241
Talarico S, Zhang L, Marrs CF et al (2008) Mycobacterium tuberculosis PE_PGRS16 and PE_PGRS26 genetic polymorphism among clinical isolates. Tuberculosis (Edinb) 88(4):283–294
Dheenadhayalan V, Delogu G, Sanguinetti M et al (2006) Variable expression patterns of Mycobacterium tuberculosis PE_PGRS genes: evidence that PE_PGRS16 and PE_PGRS26 are inversely regulated in vivo. J Bacteriol 188(10):3721–3725
Banu S, Honoré N, Saint-Joanis B et al (2002) Are the PE-PGRS proteins of Mycobacterium tuberculosis variable surface antigens? Mol Microbiol 44(1):9–19
Singh VK, Berry L, Bernut A et al (2016) A unique PE_PGRS protein inhibiting host cell cytosolic defenses and sustaining full virulence of Mycobacterium marinum in multiple hosts. Cell Microbiol 18(11):1489–1507
Ramakrishnan L, Federspiel NA, Falkow S (2000) Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288(5470):1436–1439
Srivastava V, Jain A, Srivastava BS et al (2008) Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. Tuberculosis 88(3):171–177
Meena, LS (2014) An overview to understand the role of PE_PGRS family proteins in Mycobacterium tuberculosis H 37 Rv and their potential as new drug targets. Biotechnol Appl BioChem 62(2):145–153
Lakshminarayan H, Narayanan S, Bach H et al (2008) Molecular cloning and biochemical characterization of a serine threonine protein kinase, PknL, from Mycobacterium tuberculosis. Protein Expr Purif 58(2):309–317
Deng W, Li W, Zeng J et al (2014) Mycobacterium tuberculosis PPE family protein Rv1808 manipulates cytokines profile via co-activation of MAPK and NF-kappaB signaling pathways. Cell Physiol Biochem 33(2):273–288
Mazandu GK, Mulder NJ (2012) Function prediction and analysis of Mycobacterium tuberculosis hypothetical proteins. Int J Mol Sci 13(6):7283–7302
Bottai D, Di Luca M, Majlessi L et al (2012) Disruption of the ESX-5 system of Mycobacterium tuberculosis causes loss of PPE protein secretion, reduction of cell wall integrity and strong attenuation. Mol Microbiol 83(6):1195–1209
Tiwari BM, Kannan N, Vemu L et al (2012) The Mycobacterium tuberculosis PE proteins Rv0285 and Rv1386 modulate innate immunity and mediate bacillary survival in macrophages. Plos ONE 7(12):e51686–e51686
Singh SK, Tripathi DK, Singh PK et al (2012) Protective and survival efficacies of Rv0160c protein in murine model of Mycobacterium tuberculosis. Appl Microbiol Biotechnol 97(13):5825–5837
Gupta D, Sharma S, Singhal J et al (2010) Suppression of TLR2-induced IL-12, reactive oxygen species, and inducible nitric oxide synthase expression by Mycobacterium tuberculosis antigens expressed inside macrophages during the course of infection. J Immunol 184(10):5444–5455
Voskuil MI, Schnappinger D, Visconti KC et al (2003) Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198(5):705–713
Fishbein S, Wyk N, Warren RM et al (2015) Phylogeny to function: PE/PPE protein evolution and impact on Mycobacterium tuberculosis pathogenicity. Mol Microbiol 96(5):901–916
Leemans JC, Thepen T, Weijer S et al (2005) Macrophages play a dual role during pulmonary tuberculosis in mice. J Infect Dis 191(1):65–74
Flynn JL, Chan J (2003) Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr Opin Immunol 15(4):450–455
Delogu G, Brennan MJ (2001) Comparative immune response to PE and PE_PGRS antigens of Mycobacterium tuberculosis. Infect Immun 69(9):5606–5611
Dheenadhayalan V, Delogu G, Brennan MJ (2006) Expression of the PE_PGRS 33 protein in Mycobacterium smegmatis triggers necrosis in macrophages and enhanced mycobacterial survival. Microbes Infect 8(1):262–272
Cadieux N, Parra M, Cohen H et al (2011) Induction of cell death after localization to the host cell mitochondria by the Mycobacterium tuberculosis PE_PGRS33 protein. Microbiology 157(Pt 3):793–804
Chaitra MG, Shaila MS, Nayak R (2007) Evaluation of T-cell responses to peptides with MHC class I-binding motifs derived from PE_PGRS 33 protein of Mycobacterium tuberculosis. J Med Microbiol 56(Pt 4):466–474
Singh PP, Parra M, Cadieux N et al (2008) A comparative study of host response to three Mycobacterium tuberculosis PE_PGRS proteins. Microbiology 154(Pt 11):3469–3479
Chatrath S, Gupta VK, Dixit A et al (2016) PE_PGRS30 of Mycobacterium tuberculosis mediates suppression of proinflammatory immune response in macrophages through its PGRS and PE domains. Microbes Infect 18(9):536–542
Basu S, Pathak SK, Banerjee A et al (2007) Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by Toll-like receptor 2-dependent release of tumor necrosis factor-alpha. J Biol Chem 282(2):1039–1050
Campuzano J, Aguilar D, Arriaga K et al (2007) The PGRS domain of Mycobacterium tuberculosis PE_PGRS Rv1759c antigen is an efficient subunit vaccine to prevent reactivation in a murine model of chronic tuberculosis. Vaccine 25(18):3722–3729
Jang S, Uematsu S, Akira S et al (2004) IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J Immunol 173(5):3392–3397
Fremond CM, Togbe D, Doz E et al (2007) IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol 179(2):1178–1189
Bordón J, Plankey MW, Young M et al (2011) Lower levels of interleukin-12 precede the development of tuberculosis among HIV-infected women. Cytokine 56(2):325–331
Cooper AM, Mayer-Barber KD, Sher A et al (2011) Role of innate cytokines in mycobacterial infection. Mucosal Immunol 4(3):252–260
Huang Y, Wang Y, Bai Y et al (2010) Expression of PE_PGRS 62 protein in Mycobacterium smegmatis decrease mRNA expression of proinflammatory cytokines IL-1beta, IL-6 in macrophages. Mol Cell Biochem 340(1–2):223–229
Li J, Chai QY, Zhang Y et al (2015) Mycobacterium tuberculosis Mce3E suppresses host innate immune responses by targeting ERK1/2 signaling. J Immunol 194(8):3756–3767
Muttucumaru DGN, Smith DA, McMinn EJ et al (2011) Mycobacterium tuberculosis Rv0198c, a putative matrix metalloprotease is involved in pathogenicity. Tuberculosis (Edinb) 91(2):111–116
Acknowledgements
This work was supported by National Natural Science Foundation (Grant Nos. 81371851, 81071316, 81271882, 81301394), National Key Research and Development Program (2016YFC0502304), New Century Excellent Talents in Universities (Grant No. NCET-11-0703), The Fundamental Research Funds for the Central Universities (Grant No. XDJK2016E093), The Chongqing Municipal Committee of Education for postgraduates innovation program (Grant No. CYS16073). The Fundamental Research Funds for the Central Universities (Grant Nos. XDJK2016E093, XDJK2016D055, XDJK2012D007), The Chongqing Municipal Committee of Education for postgraduates innovation program (Grant No. CYS16073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Wenmin Yang and Wanyan Deng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, W., Deng, W., Zeng, J. et al. Mycobacterium tuberculosis PE_PGRS18 enhances the intracellular survival of M. smegmatis via altering host macrophage cytokine profiling and attenuating the cell apoptosis. Apoptosis 22, 502–509 (2017). https://doi.org/10.1007/s10495-016-1336-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1336-0