Abstract
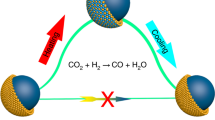
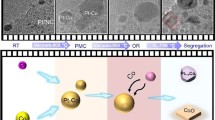
Alloying metals to form intermetallics has been proven effective in tuning the chemical properties of metal-based catalysts. However, intermetallic alloys can undergo structural and chemical transformations under reactive conditions, leading to changes in their catalytic function. Elucidating and understanding these transformations are crucial for establishing relevant structure-performance relationships and for the rational design of alloy-based catalysts. In this work, we used CuZn alloy nanoparticles (NPs) as a model material system and employed in situ transmission electron microscopy (TEM) to investigate the structural and chemical changes of CuZn NPs under H2, O2 and their mixture. Our results show how CuZn NPs undergo sequential transformations in the gas mixture at elevated temperatures, starting with gradual leaching and segregation of Zn, followed by oxidation at the NP surface. The remaining copper at the core of particles can then engage in dynamic behavior, eventually freeing itself from the zinc oxide shell. The structural dynamics arises from an oscillatory phase transition between Cu and Cu2O and is correlated with the catalytic water formation, as confirmed by in situ mass spectrometry (MS). Under pure H2 or O2 atmosphere, we observe different structural evolution pathways and final chemical states of CuZn NPs compared to those in the gas mixture. These results clearly demonstrate that the chemical state of alloy NPs can vary considerably under reactive redox atmospheres, particularly for those containing elements with distinct redox properties, necessitating the use of in situ or detailed ex situ characterizations to gain relevant insights into the states of intermetallic alloy-based catalysts and structure-activity relationships.

Similar content being viewed by others
References
Ferrando, R.; Jellinek, J.; Johnston, R. L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910.
Luneau, M.; Guan, E. J.; Chen, W.; Foucher, A. C.; Marcella, N.; Shirman, T.; Verbart, D. M. A.; Aizenberg, J.; Aizenberg, M.; Stach, E. A. et al. Enhancing catalytic performance of dilute metal alloy nanomaterials. Commun. Chem. 2020, 3, 46.
Gilroy, K. D.; Ruditskiy, A.; Peng, H. C.; Qin, D.; Xia, Y. N. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472.
Luneau, M.; Lim, J. S.; Patel, D. A.; Sykes, E. C. H.; Friend, C. M.; Sautet, P. Guidelines to achieving high selectivity for the hydrogenation of α,β-unsaturated aldehydes with bimetallic and dilute alloy catalysts: A review. Chem. Rev. 2020, 120, 12834–12872.
Chia, S. R.; Nomanbhay, S.; Ong, M. Y.; Chew, K. W.; Khoo, K. S.; Karimi-Maleh, H.; Show, P. L. Recent development of renewable diesel production using bimetallic catalysts. Front. Energy Res. 2021, 9, 769485.
Luo, L. L.; Li, L.; Schreiber, D. K.; He, Y.; Baer, D. R.; Bruemmer, S. M.; Wang, C. M. Deciphering atomistic mechanisms of the gassolid interfacial reaction during alloy oxidation. Sci. Adv. 2020, 6, eaay8491.
Kim, J.; Choi, H.; Kim, D.; Park, J. Y. Operando surface studies on metal-oxide interfaces of bimetal and mixed catalysts. ACS Catal. 2021, 11, 8645–8677.
Zhang, Y. B.; Pan, J. A.; Gong, G.; Song, R. X.; Yuan, Y.; Li, M. Z.; Hu, W. F.; Fan, P. C.; Yuan, L. X.; Wang, L. L. In situ surface reconstruction of catalysts for enhanced hydrogen evolution. Catalysts 2023, 13, 120
van den Berg, R.; Prieto, G.; Korpershoek, G.; van der Wal, L. I.; van Bunningen, A. J.; Lœgsgaard-Jørgensen, S.; de Jongh, P. E.; de Jong, K. P. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis. Nat. Commun. 2016, 7, 13057.
Renzas, J. R.; Huang, W. Y.; Zhang, Y. W.; Grass, M. E.; Hoang, D. T.; Alayoglu, S.; Butcher, D. R.; Tao, F.; Liu, Z.; Somorjai, G. A. Rh1-xPdx nanoparticle composition dependence in CO oxidation by oxygen: Catalytic activity enhancement in bimetallic systems. Phys. Chem. Chem. Phys. 2011, 13, 2556–2562.
Großmann, D.; Klementiev, K.; Sinev, I.; Gr⋼nert, W. Surface alloy or metal-cation interaction-the state of Zn promoting the active Cu sites in methanol synthesis catalysts. CeemCatCeem 2017, 9, 365–372.
Wang, D. L.; Xin, H. L.; Hovden, R.; Wang, H. S.; Yu, Y. C.; Muller, D. A.; Disalvo, F. J.; Abruna, H. D. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87.
Holse, C.; Elkjør, C. F.; Nierhoff, A.; Sehested, J.; Chorkendorff, I.; Helveg, S.; Nielsen, J. H. Dynamic behavior of CuZn nanoparticles under oxidizing and reducing conditions. J. Phys. Chem. C 2015, 119, 2804–2812.
Simonovis, J. P.; Hunt, A.; Palomino, R. M.; Senanayake, S. D.; Waluyo, I. Enhanced stability of Pt-Cu single-atom alloy catalysts: In situ characterization of the Pt/Cu(111) surface in an ambient pressure of CO. J. Phys. Chem. C 2018, 122, 4488–4495.
Tao, F.; Grass, M. E.; Zhang, Y. W.; Butcher, D. R.; Renzas, J. R.; Liu, Z.; Chung, J. Y.; Mun, B. S.; Salmeron, M.; Somorjai, G. A. Reaction-driven restructuring of Rh-Pd and Pt-Pd core-shell nanoparticles. Science 2008, 322, 932–934.
Chee, S. W.; Lunkenbein, T.; Schlögl, R.; Cuenya, B. R. In situ and operando electron microscopy in heterogeneous catalysis-insights into multi-scale chemical dynamics. J. Phys.: Condens. Matter 2021, 33, 153001.
Tang, M.; Yuan, W. T.; Ou, Y.; Li, G. X.; You, R. Y.; Li, S. D.; Yang, H. S.; Zhang, Z.; Wang, Y. Recent progresses on structural reconstruction of nanosized metal catalysts via controlled-atmosphere transmission electron microscopy: A review. ACS Catal. 2020, 10, 14419–14450.
Tao, F.; Crozier, P. A. Atomic- scale observations of catalyst structures under reaction conditions and during catalysis. Chem. Rev. 2016, 116, 3487–3539.
Kalz, K. F.; Kraehnert, R.; Dvoyashkin, M.; Dittmeyer, R.; Gläser, R.; Krewer, U.; Reuter, K.; Grunwaldt, J. D. Future challenges in heterogeneous catalysis: Understanding catalysts under dynamic reaction conditions. ChemCatChem 2017, 9, 17–29.
Newton, M. A. Dynamic adsorbate/reaction induced structural change of supported metal nanoparticles: Heterogeneous catalysis and beyond. Chem. Soc. Rev. 2008, 37, 2644–2657.
Foucher, A. C.; Marcella, N.; Lee, J. D.; Tappero, R.; Murray, C. B.; Frenkel, A. I.; Stach, E. A. Dynamical change of valence states and structure in NiCu3 nanoparticles during redox cycling. J. Phys. Chem. C 2022, 126, 1991–2002.
Wang, C. M.; Genc, A.; Cheng, H. K.; Pullan, L.; Baer, D. R.; Bruemmer, S. M. In situ TEM visualization of vacancy injection and chemical partition during oxidation of Ni-Cr nanoparticles. Sci. Rep. 2014, 4, 3683
Guan, Y. Y.; Liu, Y. T.; Ren, Q. Y.; Dong, Z. J.; Luo, L. L. Oxidation-induced phase separation of carbon-supported CuAu nanoparticles for electrochemical reduction of CO2. Nano Res. 2023, 16, 2119–2125.
Luo, L. L.; Su, M.; Yan, P. F.; Zou, L. F.; Schreiber, D. K.; Baer, D. R.; Zhu, Z. H.; Zhou, G. W.; Wang, Y. T.; Bruemmer, S. M. et al. Atomic origins of water-vapour-promoted alloy oxidation. Nat. Mater. 2018, 17, 514–518.
Zhang, X. B.; Han, S. B.; Zhu, B. E.; Zhang, G. H.; Li, X. Y.; Gao, Y.; Wu, Z. X.; Yang, B.; Liu, Y. F.; Baaziz, W. et al. Author correction: Reversible loss of core-shell structure for Ni-Au bimetallic nanoparticles during CO2 hydrogenation. Nat. Catal. 2021, 4, 180.
Moscu, A.; Theodoridi, C.; Cardenas, L.; Thieuleux, C.; Motta-Meira, D.; Agostini, G.; Schuurman, Y.; Meunier, F. CO dissociation on Pt-Sn nanoparticles triggers Sn oxidation and alloy segregation. J. Catal. 2018, 359, 76–81.
Gao, F.; Wang, Y. L.; Goodman, D. W. CO oxidation over AuPd(100) from ultrahigh vacuum to near-atmospheric pressures: CO adsorption-induced surface segregation and reaction kinetics. J. Phys. Chem. C 2009, 113, 14993–15000.
Yasuhara, A.; Homma, M.; Sannomiya, T. In situ observation of structural and optical changes of phase-separated Ag-Cu nanoparticles during a dewetting process via transmission electron microscopy. ACS Appl. Mater. Interfaces 2022, 14, 35020–35026.
Chen, P. C.; Gao, M. Y.; Yu, S.; Jin, J. B.; Song, C. Y.; Salmeron, M.; Scott, M. C.; Yang, P. D. Revealing the phase separation behavior of thermodynamically immiscible elements in a nanoparticle. Nano Lett. 2021, 21, 6684–6689.
Zafeiratos, S.; Piccinin, S.; Teschner, D. Alloys in catalysis: Phase separation and surface segregation phenomena in response to the reactive environment. Catal. Sci. Technol. 2012, 2, 1787–1801.
Tisseraud, C.; Comminges, C.; Habrioux, A.; Pronier, S.; Pouilloux, Y.; Le Valant, A. Cu- ZnO catalysts for CO2 hydrogenation to methanol: Morphology change induced by ZnO lixiviation and its impact on the active phase formation. Mol. Catal. 2018, 446, 98–105.
Frey, H.; Beck, A.; Huang, X.; van Bokhoven, J. A.; Willinger, M. G. Dynamic interplay between metal nanoparticles and oxide support under redox conditions. Science 2022, 376, 982–987.
Yuan, W. T.; Zhu, B. E.; Fang, K.; Li, X. Y.; Hansen, T. W.; Ou, Y.; Yang, H. S.; Wagner, J. B.; Gao, Y.; Wang, Y. et al. In situ manipulation of the active Au-TiO2 interface with atomic precision during CO oxidation. Science 2021, 371, 517–521
He, B. W.; Zhang, Y. X.; Liu, X.; Chen, L. W. In situ transmission electron microscope techniques for heterogeneous catalysis. ChemCatChem 2020, 12, 1853–1872
Huang, X.; Beck, A.; Fedorov, A.; Frey, H.; Zhang, B. S.; Klötzer, B.; van Bokhoven, J. A.; Copéret, C.; Willinger, M. G. Visualizing structural and chemical transformations of an industrial Cu/ZnO/Al2O3 pre- catalyst during activation and CO2 reduction. ChemCatChem 2022, 14, e202201280.
Niu, Y. M.; Wang, Y. Z.; Chen, J. N.; Li, S. Y.; Huang, X.; Willinger, M. G.; Zhang, W.; Liu, Y. F.; Zhang, B. S. Patterning the consecutive Pd3 to Pd1 on Pd2Ga surface via temperature-promoted reactive metal-support interaction. Sci. Adv. 2022, 8, eabq5751.
Li, X.; Cheng, S. B.; He, Y. H.; Qian, L. X.; Zakharov, D.; Wu, G.; Shan, C. X.; Zhang, L.; Su, D. Revealing the dynamics of the alloying and segregation of Pt-Co nanoparticles via in-situ environmental transmission electron microscopy. Nano Res. 2023, 16, 3055–3062.
Liu, P. P.; Klyushin, A.; Chandramathy Surendran, P.; Fedorov, A.; Xie, W. J.; Zeng, C. B.; Huang, X. Carbon encapsulation of supported metallic Iridium nanoparticles: An in situ transmission electron microscopy study and implications for hydrogen evolution reaction. ACS Nano 2023, 17, 24395–24403.
Vitos, L.; Ruban, A. V.; Skriver, H. L.; Kollär, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202.
Takrori, F. M.; Ayyad, A. Surface energy of metal alloy nanoparticles. Appl. Surf. Sci. 2017, 401, 65–68.
Kattel, S.; Ramirez, P. J.; Chen, J. G.; Rodriguez, J. A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299.
Divins, N. J.; Kordus, D.; Timoshenko, J.; Sinev, I.; Zegkinoglou, I.; Bergmann, A.; Chee, S. W.; Widrinna, S.; Karslioğlu, O.; Mistry, H. et al. Operando high-pressure investigation of size-controlled CuZn catalysts for the methanol synthesis reaction. Nat. Commun. 2021, 12, 1435.
Behrens, M.; Studt, F.; Kasatkin, I.; K⋼hl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B. L. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897.
Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjør, C. F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Scincee 2016, 352, 969–974.
Lunkenbein, T.; Schumann, J.; Behrens, M.; Schlögl, R.; Willinger, M. G. Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions. Angew. Chem. 2015, 127, 4627–4631.
Beck, A.; Zabilskiy, M.; Newton, M. A.; Safonova, O.; Willinger, M. G.; van Bokhoven, J. A. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 2021, 4, 488–497.
Bhaskar, S. P.; Jagirdar, B. R. A journey from bulk brass to nanobrass: A comprehensive study showing structural evolution of various Cu/Zn bimetallic nanophases from the vaporization of brass. J. Alloys Compd. 2017, 694, 581–595.
LaGrow, A. P.; Ward, M. R.; Lloyd, D. C.; Gai, P. L.; Boyes, E. D. Visualizing the Cu/Cu2O interface transition in nanoparticles with environmental scanning transmission electron microscopy. J. Am. Chem. Soc. 2017, 139, 179–185.
Dong, Z. J.; Liu, W.; Zhang, L. F.; Wang, S. B.; Luo, L. L. Structural evolution of Cu/ZnO catalysts during water-gas shift reaction: An in situ transmission electron microscopy study. ACS Appl. Mater. Interfaces 2021, 13, 41707–41714.
Huang, X.; Jones, T.; Fedorov, A.; Farra, R.; Copéret, C.; Schlögl, R.; Willinger, M. G. Phase coexistence and structural dynamics of redox metal catalysts revealed by operando TEM. Adv. Mater. 2021, 33, 2101772.
Fan, H. J.; Gösele, U.; Zacharias, M. Formation of nanotubes and hollow nanoparticles based on Kirkendall and diffusion processes: A review. Small 2007, 3, 1660–1671.
Railsback, J. G.; Johnston-Peck, A. C.; Wang, J. W.; Tracy, J. B. Size-dependent nanoscale Kirkendall effect during the oxidation of nickel nanoparticles. ACS Nano 2010, 4, 1913–1920.
You, R. Y.; Ou, Y.; Qi, R.; Yu, J.; Wang, F.; Jiang, Y.; Zou, S. H.; Han, Z. K.; Yuan, W. T.; Yang, H. S. et al. Revealing temperature-dependent oxidation dynamics of Ni nanoparticles via ambient pressure transmission electron microscopy. Nano Lett. 2023, 23, 7260–7266.
Ghijsen, J.; Tjeng, L. H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G. A.; Czyzyk, M. T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330.
Mosrati, J.; Ishida, T.; Mac, H.; Al-Yusufi, M.; Honma, T.; Parliniska-Wojtan, M.; Kobayashi, Y.; Klyushin, A.; Murayama, T.; Abdel-Mageed, A. M. Low- temperature hydrogenation of CO2 to methanol in water on ZnO-supported CuAu nanoalloys. Angew. Chem., Int. Ed. 2023, 62, e202311340.
Jirka, I. An ESCA study of copper clusters on carbon. Surf. Sci. 1990, 232, 307–315.
Biesinger, M. C.; Lau, L. W. M.; Gerson, A. R.; Smart, R. S. C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898.
Biesinger, M. C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334.
Biesinger, M. C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681.
Hantsche, H. High resolution XPS of organic polymers, the scienta ESCA300 database. By G. Beamson and D. Briggs, Wiley, Chichester 1992, 295 pp., hardcover, £ 65.00, ISBN 0-471-93592-1. Adv. Mater. 1993, 5, 778.
Kamarulzaman, N.; Kasim, M. F.; Chayed, N. F. Elucidation of the highest valence band and lowest conduction band shifts using XPS for ZnO and Zn0.99Cu0.01O band gap changes. Results Phys. 2016, 6, 217–230.
Morozov, I. G.; Belousova, O. V.; Ortega, D.; Mafina, M. K.; Kuznetcov, M. V. Structural, optical, XPS and magnetic properties of Zn particles capped by ZnO nanoparticles. J. Alloys Compd. 2015, 633, 237–245.
Acknowledgements
We acknowledge MAX IV Laboratory for time on Beamline HIPPIE under 20230099 agreements. Research conducted at MAX IV, a Swedish national user facility, is supported by the Swedish Research council under contract 2018-07152, the Swedish Governmental Agency for Innovation Systems under contract 2018-04969, and Formas under contract 2019-02496. X. H. thanks 1000 talent youth project, Fuzhou University and Qingyuan Innovation Laboratory for the financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2024_6538_MOESM1_ESM.pdf
Structural and chemical transformations of CuZn alloy nanoparticles under reactive redox atmospheres: An in situ TEM study
Rights and permissions
About this article
Cite this article
Yue, S., Li, Q., Zeng, C. et al. Structural and chemical transformations of CuZn alloy nanoparticles under reactive redox atmospheres: An in situ TEM study. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6538-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6538-0