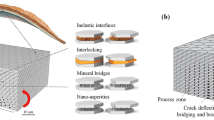
Abstract
Biomineralization process regulates the growth of inorganic minerals by complex molecules, proteins, and cells, endowing bio-materials with marvels structures and excellent properties. The intricate structures and compositions found in biominerals have inspired scientists to design and synthesize numerous artificial biomimetic materials. The methodology for controlling the formation of inorganics plays a pivotal role in achieving biomimetic structures and compositions. However, the current approach predominantly relies on the classical nucleation theory, which hinders the precise preparation of inorganic materials by replicating the biomineralization strategy. Recently, the development of “inorganic ionic polymerization” strategy has enabled us to regulate the arrangement of inorganic ions from solution to solid phase, which establishes an artificial way to produce inorganic materials analogous to the biomineralization process. Based on inorganic ionic polymerization, a series of achievements have been realized for the biomimetic preparation, including moldable construction of inorganic materials, hard tissue regeneration, and high-performance biomimetic materials. Moreover, the utilization of inorganic ionic polymerization has also facilitated the production of numerous advanced materials, including novel structures that exceed the current knowledge of materials science. The inorganic ionic polymerization system provides new artificial strategies and methodologies for the controllable synthesis of inorganics, which mimics the biomineralization process, paving the way for the future development of more high-performance materials.

Similar content being viewed by others
References
Yao, S. S.; Jin, B.; Liu, Z. M.; Shao, C. Y.; Zhao, R. B.; Wang, X. Y.; Tang, R. K. Biomineralization: From material tactics to biological strategy. Adv. Mater. 2017, 29, 1605903.
Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822.
Wallace, A. F.; Hedges, L. O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J. D.; Waychunas, G. A.; Whitelam, S.; Banfield, J. F.; De Yoreo, J. J. Microscopic evidence for liquid–liquid separation in supersaturated CaCO3 solutions. Science 2013, 341, 885–889.
Mahamid, J.; Aichmayer, B.; Shimoni, E.; Ziblat, R.; Li, C. H.; Siegel, S.; Paris, O.; Fratzl, P.; Weiner, S.; Addadi, L. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc. Natl. Acad. Sci. USA 2010, 107, 6316–6321.
Weiss, I. M.; Tuross, N.; Addadi, L.; Weiner, S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zool. 2002, 293, 478–491.
Yao, H. B.; Ge, J.; Mao, L. B.; Yan, Y. X.; Yu, S. H. 25th anniversary article: Artificial carbonate nanocrystals and layered structural nanocomposites inspired by nacre: Synthesis, fabrication and applications. Adv. Mater. 2014, 26, 163–187.
He, W. X.; Rajasekharan, A. K.; Tehrani-Bagha, A. R.; Andersson, M. Mesoscopically ordered bone-mimetic nanocomposites. Adv. Mater. 2015, 27, 2260–2264.
Liu, Y.; Luo, D.; Wang, T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small 2016, 12, 4611–4632.
Palmer, L. C.; Newcomb, C. J.; Kaltz, S. R.; Spoerke, E. D.; Stupp, S. I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783.
Shavit, K.; Wagner, A.; Schertel, L.; Farstey, V.; Akkaynak, D.; Zhang, G.; Upcher, A.; Sagi, A.; Yallapragada, V. J.; Haataja, J. et al. A tunable reflector enabling crustaceans to see but not be seen. Science 2023, 379, 695–700.
Teyssier, J.; Saenko, S. V.; van der Marel, D.; Milinkovitch, M. C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 6368.
Canal, C.; Pastorino, D.; Mestres, G.; Schuler, P.; Ginebra, M. P. Relevance of microstructure for the early antibiotic release of fresh and pre-set calcium phosphate cements. Acta Biomater. 2013, 9, 8403–8412.
Su, Y. C.; Cockerill, I.; Zheng, Y. F.; Tang, L. P.; Qin, Y. X.; Zhu, D. H. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206.
Guo, P.; Liu, X. Z.; Zhang, P. H.; He, Z. Y.; Li, Z.; Alini, M.; Richards, R. G.; Grad, S.; Stoddart, M. J.; Zhou, G. Q. et al. A single-cell transcriptome of mesenchymal stromal cells to fabricate bioactive hydroxyapatite materials for bone regeneration. Bioact. Mater. 2022, 9, 281–298.
Mao, L. B.; Gao, H. L.; Yao, H. B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S. M.; Li, S. K.; Yan, Y. X.; Liu, Y. Y. et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110.
Peng, J. S.; Huang, C. J.; Cao, C.; Saiz, E.; Du, Y.; Dou, S. X.; Tomsia, A. P.; Wagner, H. D.; Jiang, L.; Cheng, Q. F. Inverse nacrelike epoxy-graphene layered nanocomposites with integration of high toughness and self-monitoring. Matter 2020, 2, 220–232.
Guan, Q. F.; Yang, H. B.; Han, Z. M.; Ling, Z. C.; Yu, S. H. An all-natural bioinspired structural material for plastic replacement. Nat. Commun. 2020, 11, 5401.
Wang, H. G.; Lu, R. J.; Yan, J.; Peng, J. S.; Tomsia, A. P.; Liang, R.; Sun, G. X.; Liu, M. J.; Jiang, L.; Cheng, Q. F. Tough and conductive nacre-inspired MXene/epoxy layered bulk nanocomposites. Angew. Chem., Int. Ed. 2023, 62, e202216874.
Yeom, B.; Sain, T.; Lacevic, N.; Bukharina, D.; Cha, S. H.; Waas, A. M.; Arruda, E. M.; Kotov, N. A. Abiotic tooth enamel. Nature 2017, 543, 95–98.
Zhao, H. W.; Liu, S. J.; Wei, Y.; Yue, Y. H.; Gao, M. R.; Li, Y. B.; Zeng, X. L.; Deng, X. L.; Kotov, N. A.; Guo, L. et al. Multiscale engineered artificial tooth enamel. Science 2022, 375, 551–556.
Liu, M. J.; Wang, S. T.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036.
Xiong, W.; Zhao, X. H.; Zhu, G. X.; Shao, C. Y.; Li, Y. L.; Ma, W. M.; Xu, X. R.; Tang, R. K. Silicification-induced cell aggregation for the sustainable production of H2 under aerobic conditions. Angew. Chem., Int. Ed. 2015, 54, 11961–11965.
Wang, G. C.; Cao, R. Y.; Chen, R.; Mo, L. J.; Han, J. F.; Wang, X. Y.; Xu, X. R.; Jiang, T.; Deng, Y. Q.; Lyu, K. et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 7619–7624.
Wang, B.; Liu, P.; Jiang, W. G.; Pan, H. H.; Xu, X. R.; Tang, R. K. Yeast cells with an artificial mineral shell: Protection and modification of living cells by biomimetic mineralization. Angew. Chem., Int. Ed. 2008, 47, 3560–3564.
Zhao, Y. Q.; Fan, M. J.; Chen, Y. N.; Liu, Z. M.; Shao, C. Y.; Jin, B.; Wang, X. Y.; Hui, L. L.; Wang, S. F.; Liao, Z. P. et al. Surface-anchored framework for generating RhD-epitope stealth red blood cells. Sci. Adv. 2020, 6, eaaw9679.
Hao, H. B.; Wu, S. P.; Lin, J. K.; Zheng, Z. T.; Zhou, Y. M.; Zhang, Y.; Guo, Q.; Tian, F. C.; Zhao, M. S.; Chen, Y. et al. Immunization against Zika by entrapping live virus in a subcutaneous self-adjuvanting hydrogel. Nat. Biomed. Egg., in press, https://doi.org/10.1038/s41551-023-01014-4.
Ping, H.; Wagermaier, W.; Horbelt, N.; Scoppola, E.; Li, C. G.; Werner, P.; Fu, Z. Y.; Fratzl, P. Mineralization generates megapascal contractile stresses in collagen fibrils. Science 2022, 376, 188–192.
Ma, Y. X.; Jiao, K.; Wan, Q. Q.; Li, J.; Liu, M. Y.; Zhang, Z. B.; Qin, W.; Wang, K. Y.; Wang, Y. Z.; Tay, F. R. et al. Silicified collagen scaffold induces semaphorin 3A secretion by sensory nerves to improve in-situ bone regeneration. Bioact. Mater. 2022, 9, 475–490.
Sun, J. L.; Jiao, K.; Niu, L. N.; Jiao, Y.; Song, Q.; Shen, L. J.; Tay, F. R.; Chen, J. H. Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration. Biomaterials 2017, 113, 203–216.
Xu, Y. F.; Nudelman, F.; Eren, E. D.; Wirix, M. J. M.; Cantaert, B.; Nijhuis, W. H.; Hermida-Merino, D.; Portale, G.; Bomans, P. H. H.; Ottmann, C. et al. Intermolecular channels direct crystal orientation in mineralized collagen. Nat. Commun. 2020, 11, 5068.
Cherniukh, I.; Rainò, G.; Stöferle, T.; Burian, M.; Travesset, A.; Naumenko, D.; Amenitsch, H.; Erni, R.; Mahrt, R. F.; Bodnarchuk, M. I. et al. Perovskite-type superlattices from lead halide perovskite nanocubes. Nature 2021, 593, 535–542.
Picker, A.; Nicoleau, L.; Burghard, Z.; Bill, J.; Zlotnikov, I.; Labbez, C.; Nonat, A.; Cölfen, H. Mesocrystalline calcium silicate hydrate: A bioinspired route toward elastic concrete materials. Sci. Adv. 2017, 3, e1701216.
Santos, P. J.; Gabrys, P. A.; Zornberg, L. Z.; Lee, M. S.; Macfarlane, R. J. Macroscopic materials assembled from nanoparticle superlattices. Nature 2021, 591, 586–591.
Cölfen, H.; Antonietti, M. Mesocrystals and Nonclassical Crystallization; Wiley and Sons: Hoboken, 2008.
De Yoreo, J. J.; Gilbert, P. U. P. A.; Sommerdijk, N. A. J. M.; Penn, R. L.; Whitelam, S.; Joester, D.; Zhang, H. Z.; Rimer, J. D.; Navrotsky, A.; Banfield, J. F. et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760.
Nielsen, M. H.; Aloni, S.; De Yoreo, J. J. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 2014, 345, 1158–1162.
Li, D. S.; Nielsen, M. H.; Lee, J. R. I.; Frandsen, C.; Banfield, J. F.; De Yoreo, J. J. Direction-specific interactions control crystal growth by oriented attachment. Science 2012, 336, 1014–1018.
Lupulescu, A. I.; Rimer, J. D. In situ imaging of silicalite-1 surface growth reveals the mechanism of crystallization. Science 2014, 344, 729–732.
Mass, T.; Giuffre, A. J.; Sun, C. Y.; Stifler, C. A.; Frazier, M. J.; Neder, M.; Tamura, N.; Stan, C. V.; Marcus, M. A.; Gilbert, P. U. P. A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2017, 114, E7670–E7678.
Sun, C. Y.; Stifler, C. A.; Chopdekar, R. V.; Schmidt, C. A.; Parida, G.; Schoeppler, V.; Fordyce, B. I.; Brau, J. H.; Mass, T.; Tambutté, S. et al. From particle attachment to space-filling coral skeletons. Proc. Natl. Acad. Sci. USA 2020, 117, 30159–30170.
Thanh, N. T. K.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630.
Burton, W. K.; Cabrera, N.; Frank, F. C. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. Roy. A: Math. Phys. Eng. Sci. 1951, 243, 299–358.
Martin, J. D. Particle size is a primary determinant for sigmoidal kinetics of nanoparticle formation: A “disproof” of the Finke–Watzky (F–W) nanoparticle nucleation and growth mechanism. Chem. Mater. 2020, 32, 3651–3656.
Miyoshi, H.; Kimizuka, H.; Ishii, A.; Ogata, S. Temperature-dependent nucleation kinetics of Guinier–Preston zones in Al-Cu alloys: An atomistic kinetic Monte Carlo and classical nucleation theory approach. Acta Mater. 2019, 179, 262–272.
Zeglinski, J.; Kuhs, M.; Devi, K. R.; Khamar, D.; Hegarty, A. C.; Thompson, D.; Rasmuson, Å. C. Probing crystal nucleation of fenoxycarb from solution through the effect of solvent. Cryst. Growth Des. 2019, 19, 2037–2049.
Kim, D.; Lee, B.; Thomopoulos, S.; Jun, Y. S. The role of confined collagen geometry in decreasing nucleation energy barriers to intrafibrillar mineralization. Nat. Commun. 2018, 9, 962.
Yuan, K.; Starchenko, V.; Rampal, N.; Yang, F. C.; Yang, X. G.; Xiao, X. H.; Lee, W. K.; Stack, A. G. Opposing effects of impurity ion Sr2+ on the heterogeneous nucleation and growth of barite (BaSO4). Cryst. Growth Des. 2021, 21, 5828–5839.
Lahiri, J.; Xu, G. F.; Dabbs, D. M.; Yao, N.; Aksay, I. A.; Groves, J. T. Porphyrin amphiphiles as templates for the nucleation of calcium carbonate. J. Am. Chem. Soc. 1997, 119, 5449–5450.
Noorduin, W. L.; Grinthal, A.; Mahadevan, L.; Aizenberg, J. Rationally designed complex, hierarchical microarchitectures. Science 2013, 340, 832–837.
Kaplan, C. N.; Noorduin, W. L.; Li, L.; Sadza, R.; Folkertsma, L.; Aizenberg, J.; Mahadevan, L. Controlled growth and form of precipitating microsculptures. Science 2017, 355, 1395–1399.
Banfield, J. F.; Welch, S. A.; Zhang, H. Z.; Ebert, T. T.; Penn, R. L. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 2000, 289, 751–754.
Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101.
Gilbert, P. U. P. A.; Porter, S. M.; Sun, C. Y.; Xiao, S. H.; Gibson, B. M.; Shenkar, N.; Knoll, A. H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. USA 2019, 116, 17659–17665.
Gower, L. B.; Odom, D. J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734.
Xu, Y. F.; Tijssen, K. C. H.; Bomans, P. H. H.; Akiva, A.; Friedrich, H.; Kentgens, A. P. M.; Sommerdijk, N. A. J. M. Microscopic structure of the polymer-induced liquid precursor for calcium carbonate. Nat. Commun. 2018, 9, 2582.
Jiang, Y.; Gower, L.; Volkmer, D.; Cölfen, H. Hierarchical dl-glutamic acid microspheres from polymer-induced liquid precursors. Cryst. Growth Des. 2011, 11, 3243–3249.
Nudelman, F.; Pieterse, K.; George, A.; Bomans, P. H. H.; Friedrich, H.; Brylka, L. J.; Hilbers, P. A.; de With, G.; Sommerdijk, N. A. J. M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009.
Sun, S. T.; Mao, L. B.; Lei, Z. Y.; Yu, S. H.; Cölfen, H. Hydrogels from amorphous calcium carbonate and polyacrylic acid: Bio-inspired materials for “mineral plastics”. Angew. Chem., Int. Ed. 2016, 55, 11765–11769.
Yao, S. S.; Lin, X. F.; Xu, Y. F.; Chen, Y. W.; Qiu, P. C.; Shao, C. Y.; Jin, B.; Mu, Z.; Sommerdijk, N. A. J. M.; Tang, R. K. Osteoporotic bone recovery by a highly bone-inductive calcium phosphate polymer-induced liquid-precursor. Adv. Sci. 2019, 6, 1900683.
Zhu, G. M.; Sushko, M. L.; Loring, J. S.; Legg, B. A.; Song, M.; Soltis, J. A.; Huang, X. P.; Rosso, K. M.; De Yoreo, J. J. Self-similar mesocrystals form via interface-driven nucleation and assembly. Nature 2021, 590, 416–422.
Liu, Z. M.; Pan, H. H.; Zhu, G. X.; Li, Y. L.; Tao, J. H.; Jin, B.; Tang, R. K. Realignment of nanocrystal aggregates into single crystals as a result of inherent surface stress. Angew. Chem., Int. Ed. 2016, 55, 12836–12840.
Choudhary, M. K.; Kumar, M.; Rimer, J. D. Regulating nonclassical pathways of silicalite-1 crystallization through controlled evolution of amorphous precursors. Angew. Chem., Int. Ed. 2019, 58, 15712–15716.
Liao, H. G.; Zheng, H. M. Liquid cell transmission electron microscopy study of platinum iron nanocrystal growth and shape evolution. J. Am. Chem. Soc. 2013, 135, 5038–5043.
Kahil, K.; Weiner, S.; Addadi, L.; Gal, A. Ion pathways in biomineralization: Perspectives on uptake, transport, and deposition of calcium, carbonate, and phosphate. J. Am. Chem. Soc. 2021, 143, 21100–21112.
Pouget, E. M.; Bomans, P. H. H.; Goos, J. A. C. M.; Frederik, P. M.; de With, G.; Sommerdijk, N. A. J. M. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science 2009, 323, 1455–1458.
Demichelis, R.; Raiteri, P.; Gale, J. D.; Quigley, D.; Gebauer, D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat. Commun. 2011, 2, 590.
Dey, A.; Bomans, P. H.; Müller, F. A.; Will, J.; Frederik, P. M.; de With, G.; Sommerdijk, N. A. J. M. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014.
Habraken, W. J. E. M.; Tao, J. H.; Brylka, L. J.; Friedrich, H.; Bertinetti, L.; Schenk, A. S.; Verch, A.; Dmitrovic, V.; Bomans, P. H. H.; Frederik, P. M. et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 2013, 4, 1507.
Smeets, P. J. M.; Finney, A. R.; Habraken, W. J. E. M.; Nudelman, F.; Friedrich, H.; Laven, J.; De Yoreo, J. J.; Rodger, P. M.; Sommerdijk, N. A. J. M. A classical view on nonclassical nucleation. Proc. Natl. Acad. Sci. USA 2017, 114, E7882–E7890.
Henzler, K.; Fetisov, E. O.; Galib, M.; Baer, M. D.; Legg, B. A.; Borca, C.; Xto, J. M.; Pin, S.; Fulton, J. L.; Schenter, G. K. et al. Supersaturated calcium carbonate solutions are classical. Sci. Adv. 2018, 4, eaao6283.
Penn, R. L.; Banfield, J. F. Imperfect oriented attachment: Dislocation generation in defect-free nanocrystals. Science 1998, 281, 969–971.
Xu, X. X.; Zhuang, J.; Wang, X. SnO2 quantum dots and quantum wires: Controllable synthesis, self-assembled 2D architectures, and gas-sensing properties. J. Am. Chem. Soc. 2008, 130, 12527–12535.
Xu, X. X.; Wang, X. Fine tuning of the sizes and phases of ZrO2 nanocrystals. Nano Res. 2001, 2, 891–902.
Zhao, Y.; Lu, Y.; Hu, Y.; Li, J. P.; Dong, L.; Lin, L. N.; Yu, S. H. Synthesis of superparamagnetic CaCO3 mesocrystals for multistage delivery in cancer therapy. Small 2010, 6, 2436–2442.
Du, W. X.; Liu, T. Z.; Xue, F. F.; Cai, X. J.; Chen, Q.; Zheng, Y. Y.; Chen, H. R. Fe3O4 mesocrystals with distinctive magnetothermal and nanoenzyme activity enabling self-reinforcing synergistic cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 19285–19294.
Ding, Z. P.; Zhang, D. T.; Feng, Y. M.; Zhang, F.; Chen, L. B.; Du, Y.; Ivey, D. G.; Wei, W. F. Tuning anisotropic ion transport in mesocrystalline lithium orthosilicate nanostructures with preferentially exposed facets. NPG Asia Mater. 2018, 10, 606–617.
Huang, M. H.; Schilde, U.; Kumke, M.; Antonietti, M.; Cölfen, H. Polymer-induced self-assembly of small organic molecules into ultralong microbelts with electronic conductivity. J. Am. Chem. Soc. 2010, 132, 3700–3707.
Tétreault, N.; Horváth, E.; Moehl, T.; Brillet, J.; Smajda, R.; Bungener, S.; Cai, N.; Wang, P.; Zakeeruddin, S. M.; Forró, L. et al. High-efficiency solid-state dye-sensitized solar cells: Fast charge extraction through self-assembled 3D fibrous network of crystalline TiO2 nanowires. ACS Nano 2010, 4, 7644–7650.
Hu, S.; Liu, H. L.; Wang, P. P.; Wang, X. Inorganic nanostructures with sizes down to 1 nm: A macromolecule analogue. J. Am. Chem. Soc. 2013, 135, 11115–11124.
Cheng, X. J.; Zhang, S. M.; Wang, X. Cluster-nuclei coassembled one-dimensional subnanometer heteronanostructures. Nano Lett. 2021, 21, 9845–9852.
Liu, J. L.; Shi, W. X.; Ni, B.; Yang, Y.; Li, S. Z.; Zhuang, J.; Wang, X. Incorporation of clusters within inorganic materials through their addition during nucleation steps. Nat. Chem. 2011, 11, 839–845.
Liu, J. L.; Wang, S. B.; Liu, N.; Yang, D. R.; Wang, H. W.; Hu, H. S.; Zhuang, J.; Wang, X. Au-polyoxometalates A-B-A-B type copolymer-analogue sub-1 nm nanowires. Small 2021, 17, 2006260.
Zhang, S. M.; Liu, N.; Wang, H. W.; Lu, Q. C.; Shi, W. X.; Wang, X. Sub-nanometer nanobelts based on titanium dioxide/zirconium dioxide-polyoxometalate heterostructures. Adv. Mater. 2021, 33, 2100576.
Liu, J. L.; Liu, N.; Wang, H. W.; Shi, W. X.; Zhuang, J.; Wang, X. Hybrid MoO3-polyoxometallate sub-1 nm nanobelt superstructures. J. Am. Chem. Soc. 2020, 142, 17557–17563.
Liu, Q. D.; Wang, X. Fabricating sub-nanometer materials through cluster assembly. Chem. Sci. 2022, 13, 12280–12289.
Liu, Q. D.; Wang, X. Precise assembly of polyoxometalates at single-cluster levels. Angew. Chem., Int. Ed. 2023, 62, e202217764.
Liu, Q. D.; Zhang, Q. H.; Shi, W. X.; Hu, H. S.; Zhuang, J.; Wang, X. Self-assembly of polyoxometalate clusters into two-dimensional clusterphene structures featuring hexagonal pores. Nat. Chem. 2022, 14, 433–440.
Zhang, S. M.; Shi, W. X.; Wang, X. Locking volatile organic molecules by subnanometer inorganic nanowire-based organogels. Science 2022, 377, 100–104.
Fang, W. F.; Tang, R. K.; Liu, Z. M. Polymerization and crosslinking of inorganic ionic oligomers for material construction. Acta. Polym. Sin. 2021, 52, 617–633.
Howard-Fabretto, L.; Andersson, G. G. Metal clusters on semiconductor surfaces and application in catalysis with a focus on Au and Ru. Adv. Mater. 2020, 32, 1904122.
Lu, Y. Z.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623.
Lang, R.; Du, X. R.; Huang, Y. K.; Jiang, X. Z.; Zhang, Q.; Guo, Y. L.; Liu, K. P.; Qiao, B. T.; Wang, A. Q.; Zhang, T. Single-atom catalysts based on the metal-oxide interaction. Chem. Rev. 2020, 120, 11986–12043.
Devan, R. S.; Patil, R. A.; Lin, J. H.; Ma, Y. R. One-dimensional metal-oxide nanostructures: Recent developments in synthesis, characterization, and applications. Adv. Funct. Mater. 2012, 22, 3326–3370.
Hanikel, N.; Pei, X. K.; Chheda, S.; Lyu, H.; Jeong, W.; Sauer, J.; Gagliardi, L.; Yaghi, O. M. Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting. Science 2021, 374, 454–459.
Mo, Z. W.; Zhou, H. L.; Zhou, D. D.; Lin, R. B.; Liao, P. Q.; He, C. T.; Zhang, W. X.; Chen, X. M.; Zhang, J. P. Mesoporous metal-organic frameworks with exceptionally high working capacities for adsorption heat transformation. Adv. Mater. 2018, 30, 1704350.
Li, Z. J.; Jiang, F. L.; Yu, M. X.; Li, S. C.; Chen, L.; Hong, M. C. Achieving gas pressure-dependent luminescence from an AIEgen-based metal-organic framework. Nat. Commun. 2022, 13, 2142.
Daigle, J. C.; Piche, L.; Claverie, J. P. Preparation of functional polyethylenes by catalytic copolymerization. Maroomeleuules 2011, 44, 1760–1762.
Kubo, M.; Kato, N.; Uno, T.; Itoh, T. Preparation of mechanically cross-linked polystyrenes. Macromolecules 2004, 37, 2762–2765.
Drumright, R. E.; Gruber, P. R.; Henton, D. E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846.
Da Róz, A. L.; Curvelo, A. A. S.; Gandini, A. Preparation and characterization of cross-linked starch polyurethanes. Carbohydr. Polym. 2009, 77, 526–529.
Tang, B. H.; Li, W. L.; Chang, Y. C.; Yuan, B.; Wu, Y. K.; Zhang, M. T.; Xu, J. F.; Li, J.; Zhang, X. A supramolecular radical dimer: High-efficiency NIR-II photothermal conversion and therapy. Angew. Chem., Int. Ed. 2019, 58, 15526–15531.
Yang, J. P.; Wang, H.; Yin, Z. H.; Zhang, S.; Xu, J. F.; Zhang, X. Emulsion interfacial polymerization of anticancer peptides: Fabricating polypeptide nanospheres with high drug-loading efficiency and enhanced anticancer activity. Sci. China Chem. 2022, 65, 2252–2259.
Yang, C. Y.; Shen, P. C.; Ou, Q.; Peng, Q.; Zhou, S. Y.; Li, J. S.; Liu, Z. R.; Zhao, Z. J.; Qin, A. J.; Shuai, Z. G. et al. Complete deciphering of the dynamic stereostructures of a single aggregation-induced emission molecule. Matter 2022, 5, 1224–1234.
Okaniwa, M.; Takeuchi, K.; Asai, M.; Ueda, M. One-pot synthesis of dendritic poly(amide-urea)s via Curtius rearrangement. 2.Synthesis and characterization of dendritic poly(amide-urea)s. Macromolecules 2002, 35, 6232–6238.
Liu, Z. M.; Shao, C. Y.; Jin, B.; Zhang, Z. S.; Zhao, Y. Q.; Xu, X. R.; Tang, R. K. Crosslinking ionic oligomers as conformable precursors to calcium carbonate. Nature 2019, 574, 394–398.
Xiao, S. J.; Edwards, S. A.; Gräter, F. A new transferable forcefield for simulating the mechanics of CaCO3 crystals. J. Phys. Chem. C 2011, 115, 20067–20075.
Yu, Y. D.; Mu, Z.; Jin, B.; Liu, Z. M.; Tang, R. K. Organic–inorganic copolymerization for a homogenous composite without an interphase boundary. Angew. Chem., Int. Ed. 2020, 59, 2071–2075.
Yu, Y. D.; He, Y.; Mu, Z.; Zhao, Y. Q.; Kong, K. R.; Liu, Z. M.; Tang, R. K. Biomimetic mineralized organic–inorganic hybrid macrofiber with spider silk-like supertoughness. Adv. Funct. Mater. 2020, 30, 1908556.
Zhang, S. H.; Nahi, O.; Chen, L.; Aslam, Z.; Kapur, N.; Kim, Y. Y.; Meldrum, F. C. Magnesium ions direct the solid-state transformation of amorphous calcium carbonate thin films to aragonite, magnesium-calcite, or dolomite. Adv. Funct. Mater. 2022, 32, 2201394.
Zou, Z. Y.; Xie, J. J.; Macías-Sánchez, E.; Fu, Z. Y. Nonclassical crystallization of amorphous calcium carbonate in the presence of phosphate ions. Cryst. Growth Des. 2021, 21, 414–423.
Bushuev, Y. G.; Finney, A. R.; Rodger, P. M. Stability and structure of hydrated amorphous calcium carbonate. Cryst. Growth Des. 2015, 15, 5269–5279.
Lu, B. Q.; Garcia, N. A.; Chevrier, D. M.; Zhang, P.; Raiteri, P.; Gale, J. D.; Gebauer, D. Short-range structure of amorphous calcium hydrogen phosphate. Cryst. Growth Des. 2019, 19, 3030–3038.
Ihli, J.; Kim, Y. Y.; Noel, E. H.; Meldrum, F. C. The effect of additives on amorphous calcium carbonate (ACC): Janus behavior in solution and the solid state. Adv. Funct. Mater. 2013, 23, 1575–1585.
Wolf, S. E.; Leiterer, J.; Pipich, V.; Barrea, R.; Emmerling, F.; Tremel, W. Strong stabilization of amorphous calcium carbonate emulsion by ovalbumin: Gaining insight into the mechanism of “polymer-induced liquid precursor” processes. J. Am. Chem. Soc. 2011, 133, 12642–12649.
Hong, M. N.; Moreland, K. T.; Chen, J. J.; Teng, H. H.; Thalmann, R.; De Yoreo, J. J. Effect of otoconial proteins fetuin A, osteopontin, and otoconin 90 on the nucleation and growth of calcite. Cryst. Growth Des. 2015, 15, 129–136.
Jiang, S. Q.; Chen, Y.; Pan, H. H.; Zhang, Y. J.; Tang, R. K. Faster nucleation at lower pH: Amorphous phase mediated nucleation kinetics. Phys. Chem. Chem. Phys. 2013, 15, 12530–12533.
Jiang, S. Q.; Pan, H. H.; Chen, Y.; Xu, X. R.; Tang, R. K. Amorphous calcium phosphate phase-mediated crystal nucleation kinetics and pathway. Faraday Discuss. 2015, 179, 451–461.
Ding, H. C.; Pan, H. H.; Xu, X. R.; Tang, R. K. Toward a detailed understanding of magnesium ions on hydroxyapatite crystallization inhibition. Cryst. Growth Des. 2014, 14, 763–769.
Yang, H.; Chai, S. Q.; Zhang, Y. Z.; Ma, Y. R. A study on the influence of sodium carbonate concentration on the synthesis of high Mg calcites. CrystEngComm 2016, 18, 157–163.
Zhang, S. H.; Nahi, O.; He, X. F.; Chen, L.; Aslam, Z.; Kapur, N.; Kim, Y. Y.; Meldrum, F. C. Local heating transforms amorphous calcium carbonate to single crystals with defined morphologies. Adv. Funct. Mater. 2022, 32, 2207019.
Liu, Z. M.; Zhang, Z. S.; Wang, Z. M.; Jin, B.; Li, D. S.; Tao, J. H.; Tang, R. K.; De Yoreo, J. J. Shape-preserving amorphous-to-crystalline transformation of CaCO3 revealed by in situ TEM. Proc. Natl. Acad. Sci. USA 2020, 117, 3397–3404.
Mu, Z.; Kong, K. R.; Jiang, K.; Dong, H. L.; Xu, X. R.; Liu, Z. M.; Tang, R. K. Pressure-driven fusion of amorphous particles into integrated monoliths. Science 2021, 372, 1466–1470.
Nassif, N.; Pinna, N.; Gehrke, N.; Antonietti, M.; Jäger, C.; Cölfen, H. Amorphous layer around aragonite platelets in nacre. Proc. Natl. Acad. Sci. USA 2005, 102, 12653–12655.
Jin, B.; Shao, C. Y.; Wang, Y. M.; Mu, Z.; Liu, Z. M.; Tang, R. K. Anisotropic epitaxial behavior in the amorphous phase-mediated hydroxyapatite crystallization process: A new understanding of orientation control. J. Phys. Chem. Lett. 2019, 10, 7611–7616.
Schmitt, V. E. M.; Kaltschmitt, M. Effect of straw proportion and Ca- and Al-containing additives on ash composition and sintering of wood-straw pellets. Fuel 2013, 109, 551–558.
Mu, Z.; Tang, R. K.; Liu, Z. M. Construction of inorganic bulks through coalescence of particle precursors. Nanomaterials 2021, 11, 241.
Wang, C. W.; Ping, W. W.; Bai, Q.; Cui, H. C.; Hensleigh, R.; Wang, R. L.; Brozena, A. H.; Xu, Z. P.; Dai, J. Q.; Pei, Y. et al. A general method to synthesize and sinter bulk ceramics in seconds. Science 2020, 368, 521–526.
Bouville, F.; Studart, A. R. Geologically-inspired strong bulk ceramics made with water at room temperature. Nat. Commun. 2017, 8, 14655.
Wang, Y. Y.; Lin, K. L.; Wu, C. T.; Liu, X. G.; Chang, J. Preparation of hierarchical enamel-like structures from nano- to macro-scale, regulated by inorganic templates derived from enamel. J. Mater. Chem. B 2015, 3, 65–71.
Mukherjee, K.; Ruan, Q. C.; Nutt, S.; Tao, J. H.; De Yoreo, J. J.; Moradian-Oldak, J. Peptide-based bioinspired approach to regrowing multilayered aprismatic enamel. ACS Omega 2018, 3, 2546–2557.
Ruan, Q. C.; Siddiqah, N.; Li, X. C.; Nutt, S.; Moradian-Oldak, J. Amelogenin-chitosan matrix for human enamel regrowth: Effects of viscosity and supersaturation degree. Connect. Tissue Res. 2014, 55, 150–154.
Li, L.; Mao, C. Y.; Wang, J. M.; Xu, X. R.; Pan, H. H.; Deng, Y.; Gu, X. H.; Tang, R. K. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: An approach to biomaterials with optimal characteristics. Adv. Mater. 2011, 23, 4695–4701.
Shao, C. Y.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y. Q.; Wu, Z. F.; Yan, L. M.; Zhang, Z. S.; Zhou, Y. C.; Pan, H. H. et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569.
Wang, C. H.; Mutalik, C.; Yougbaré, S.; Teng, N. C.; Kuo, T. R. Calcium phosphate nanoclusters for the repair of tooth enamel erosion. Nanomaterials 2022, 12, 1997.
Khvostenko, D.; Hilton, T. J.; Ferracane, J. L.; Mitchell, J. C.; Kruzic, J. J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81.
Yang, T.; Li, Y. S.; Hong, Y. B.; Chi, L.; Liu, C. Z.; Lan, Y.; Wang, Q. M.; Yu, Y. J.; Xu, Q. B.; Teng, W. The construction of biomimetic cementum through a combination of bioskiving and fluorine-containing biomineralization. Front. Bioeng. Biotechnol. 2020, 8, 341.
Sun, J.; Chen, C. Q.; Pan, H. H.; Chen, Y.; Mao, C. Y.; Wang, W.; Tang, R. K.; Gu, X. H. Biomimetic promotion of dentin remineralization using L-glutamic acid: Inspiration from biomineralization proteins. J. Mater. Chem. B 2014, 2, 4544–4553.
Saeki, K.; Chien, Y. C.; Nonomura, G.; Chin, A. F.; Habelitz, S.; Gower, L. B.; Marshall, S. J.; Marshall, G. W. Recovery after PILP remineralization of dentin lesions created with two cariogenic acids. Arch. Oral Biol. 2017, 82, 194–202.
Chen, C. Q.; Mao, C. Y.; Sun, J.; Chen, Y.; Wang, W.; Pan, H. H.; Tang, R. K.; Gu, X. H. Glutaraldehyde-induced remineralization improves the mechanical properties and biostability of dentin collagen. Mater. Sci. Eng. C 2016, 67, 657–665.
Spencer, P.; Ye, Q.; Park, J.; Topp, E. M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B. S.; Singh, V.; Sene, F. et al. Adhesive/dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003.
Yan, L. M.; Zheng, C.; Yuan, D.; Guo, Z. X.; Cui, Y. H.; Xie, Z. J.; Chen, Z.; Tang, R. K.; Liu, Z. M. Fast construction of biomimetic organic-inorganic interface by crosslinking of calcium phosphate oligomers: A strategy for instant regeneration of hard tissue. Adv. Healthcare Mater. 2022, 11, 2201161.
Kim, H.; Choi, A.; Gong, M. K.; Park, H. R.; Kim, Y. I. Effect of remineralized collagen on dentin bond strength through calcium phosphate ion clusters or metastable calcium phosphate solution. Nanomaterials 2020, 10, 2203.
Liang, K. Y.; Zhao, C. C.; Song, C. X.; Zhao, L.; Qiu, P. C.; Wang, S. Y.; Zhu, J. J.; Gong, Z.; Liu, Z. M.; Tang, R. K. et al. In situ biomimetic mineralization of bone-like hydroxyapatite in hydrogel for the acceleration of bone regeneration. ACS Appl. Mater. Interfaces 2023, 15, 292–308.
Yao, S.; Xie, Z. A.; Ye, L.; Jin, B.; Xu, Y.; Wang, M.; Yu, C.; Tang, R.; Fang, X.; Fan, S. Ultrasmall sized calcium phosphate nanoclusters based organic-inorganic biofiber for accelerated bone fracture healing. Mater. Today Nano 2023, 21, 100290.
Yu, Y. D.; Liu, Z. M.; Zhao, Q. Inorganic ionic oligomers induced organic–inorganic synergistic toughening enabling mechanical robust and recyclable nanocomposite hydrogels. Adv. Funct. Mater. 2023, 33, 2213699.
Yu, Y. D.; Kong, K. R.; Tang, R. K.; Liu, Z. M. A bioinspired ultratough composite produced by integration of inorganic ionic oligomers within polymer networks. ACS Nano 2022, 16, 7926–7936.
He, Y.; Kong, K. R.; Guo, Z. X.; Fang, W. F.; Ma, Z. Q.; Pan, H. H.; Tang, R. K.; Liu, Z. M. A highly sensitive, reversible, and bidirectional humidity actuator by calcium carbonate ionic oligomers incorporated poly(vinylidene fluoride). Adv. Funct. Mater. 2021, 31, 2101291.
Yu, Y. D.; Kong, K. R.; Mu, Z.; Liu, Z. M.; Tang, R. K. Chameleon-inspired stress-responsive multicolored ultratough films. ACS Appl. Mater. Interfaces 2020, 12, 36731–36739.
Xi, P. Y.; Quan, F. Y.; Yao, J. Y.; Xia, Y. Z.; Fang, K. J.; Jiang, Y. J. Strategy to fabricate a strong and supertough bio-Inspired fiber with organic-inorganic networks in a green and scalable Way. ACS Nano 2021, 15, 16478–16487.
Xi, P. Y.; Luo, J.; Chen, W. C.; Jiang, Y. J. Tough and strong waterborne polyurethane network combined with sub-nanoscaled calcium phosphate oligomers for protective coating. Macromol. Mater. Eng. 2022, 307, 2200181.
Zhou, Y.; Zeng, G. D.; Zhang, F. D.; Luo, J.; Li, X. N.; Li, J. Z.; Fang, Z. Toward utilization of agricultural wastes: Development of a novel keratin reinforced soybean meal-based adhesive. ACS Sustain. Chem. Eng. 2021, 9, 7630–7637.
Li, Q.; Hu, Y. L.; Zhang, B. J. Hydrophilic ZnO nanoparticle-based antimicrobial coatings for sandstone heritage conservation. ACS Appl. Nano Mater. 2021, 4, 13908–13918.
Dong, H.; Deng, M. X.; Sun, D.; Zhao, Y. T.; Liu, H.; Xie, M.; Dong, W. J.; Huang, F. Q. Amorphous lithium-phosphate-encapsulated Fe2O3 as a high-rate and long-life anode for lithium-ion batteries. ACS Appl. Energy Mater. 2022, 5, 3463–3470.
Ye, B.; Cai, M. Z.; Xie, M.; Dong, H.; Dong, W. J.; Huang, F. Q. Constructing robust cathode/electrolyte interphase for ultrastable 4.6 V LiCoO2 under ö−25 °C. ACS Appl. Mater. Interfaces 2022, 14, 19561–19568.
Dong, W. J.; Ye, B.; Cai, M. Z.; Bai, Y. Z.; Xie, M.; Sun, X. Z.; Lv, Z. R.; Huang, F. Q. Superwettable high-voltage LiCoO2 for low-temperature lithium ion batteries. ACS Energy Lett. 2023, 8, 881–888.
Ballesteros-Soberanas, J.; Hernández-Garrido, J. C.; Cerón-Carrasco, J. P.; Leyva-Pérez, A. Selective semi-hydrogenation of internal alkynes catalyzed by Pd-CaCO3 clusters. J. Catal. 2022, 408, 43–55.
Sun, D.; Bi, Q. Y.; Deng, M. X.; Jia, B. Q.; Huang, F. Q. Atomically dispersed Pd-Ru dual sites in an amorphous matrix towards efficient phenylacetylene semi-hydrogenation. Chem. Commun. 2021, 57, 5670–5673.
Jia, Z. Z.; Shen, Y. J.; Yan, T. T.; Li, H. R.; Deng, J.; Fang, J. H.; Zhang, D. S. Efficient NOx abatement over alkali-resistant catalysts via constructing durable dimeric VOx species. Environ. Sci. Technol. 2022, 56, 2647–2655.
Hou, Z. D.; Ling, C. C.; Xue, X.; Ma, C.; Fu, J. W.; Xue, Q. Z. Surface lattice reconstruction enhanced the photoresponse performance of a self-powered ZnO nanorod arrays/Si heterojunction photodetector. J. Mater. Chem. C 2020, 8, 17440–17449.
Lin, X. Q.; Chen, J.; Xu, S. X.; Mao, T. Y.; Liu, W. P.; Wu, J. Z.; Li, X. D.; Yan, J. H. Solidification of heavy metals and PCDD/Fs from municipal solid waste incineration fly ash by the polymerization of calcium carbonate oligomers. Chemosphere 2022, 288, 132420.
Chen, J.; Li, M. J.; Mao, T. Y.; Fu, C. K.; Lin, X. Q.; Li, X. D.; Yan, J. H. Effects of curing pathways and thermal-treatment temperatures on the solidification of heavy metal in fly ash by CaCO3 oligomers polymerization. J. Cleaner Prod. 2022, 362, 132526.
Chen, J.; Zhu, W. C.; Shen, Y. Z.; Fu, C. K.; Li, M. J.; Lin, X. Q.; Li, X. D.; Yan, J. H. A novel method of calcium dissolution-crystallization-polymerization for stabilization/solidification of MSWI fly ash. Chemosphere 2023, 326, 138465.
Yu, Y. D.; Guo, Z. X.; Zhao, Y. Q.; Kong, K. R.; Pan, H. H.; Xu, X. R.; Tang, R. K.; Liu, Z. M. A flexible and degradable hybrid mineral as a plastic substitute. Adv. Mater. 2022, 34, 2107523.
Fanin, N.; Kardol, P.; Farrell, M.; Kempel, A.; Ciobanu, M.; Nilsson, M. C.; Gundale, M. J.; Wardle, D. A. Effects of plant functional group removal on structure and function of soil communities across contrasting ecosystems. Ecol. Lett. 2019, 22, 1095–1103.
Gao, L. L.; Wei, C. Q.; Xu, H.; Liu, X. Y.; Siemann, E.; Lu, X. M. Latitudinal variation in the diversity and composition of various organisms associated with an exotic plant: The role of climate and plant invasion. New Phytol. 2021, 231, 1559–1569.
Fang, W. F.; Mu, Z.; He, Y.; Kong, K. R.; Jiang, K.; Tang, R. K.; Liu, Z. M. Organic-inorganic covalent-ionic molecules for elastic ceramic plastic. Nature 2023, 619, 293–299.
Acknowledgements
The authors acknowledge funding supports from the National Natural Science Foundation of China (Nos. 22022511 and 22275161), the National Key Research and Development Program of China (No. 2020YFA0710400), and the Fundamental Research Funds for the Central Universities (Nos. 2021FZZX001-04 and 2022ZJJH02-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sang, Y., Qin, K., Tang, R. et al. Inorganic ionic polymerization: From biomineralization to materials manufacturing. Nano Res. 17, 550–569 (2024). https://doi.org/10.1007/s12274-023-6033-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-6033-z