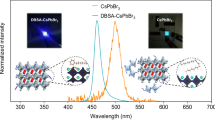
Abstract
Construction of lead halide perovskite nanocrystals (LHP NCs) heterostructures is essential to obtain highly stable photoluminescence and expand their applications. Herein, a novel self-assembly strategy combining with a solvent-free thermal-assisted synthesis and a water-triggered reaction is developed to subsequently grow BaWO4/CsPbX3/CsPb2X5 (X = Cl, Br, I) heterostructures at low nucleation temperature with high crystallinity. The as-obtained ternary BaWO4/CsPbX3/CsPb2X5 (X = Cl, Br, I) heterostructures exhibit remarkably enhanced panchromatic emission and ultrastable luminescence ascribing to the low-defect growth based on lattice matching. Stable white light-emitting diodes (WLEDs) have been constructed with a high correlated color temperature (CCT) of 7225 K and luminous efficiency of 74.4 lm·W−1. Ln3+-doped BaWO4/CsPbX3/CsPb2X5 (Ln3+ = Eu3+, Tb3+, Dy3+, Sm3+, Yb3+/Er3+) nanocomposites are further designed with excitation-dependent photoluminescence and thermochromic properties, making them excellent candidates for high-level anti-counterfeiting and encryption. This work offers a green and universal approach in assembling CsPbX3 (X = Cl, Br, I) on lattice-matched tungstate with adjustable panchromatic emission for versatile optical applications.

Similar content being viewed by others
References
Xiao, Z. W.; Song, Z. N.; Yan, Y. F. From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 2019, 31, 1803792.
Wang, H. R.; Zhang, X. Y.; Wu, Q. Q.; Cao, F.; Yang, D. W.; Shang, Y. Q.; Ning, Z. J.; Zhang, W.; Zheng, W. T.; Yan, Y. F. et al. Trifluoroacetate induced small-grained CsPbBr3 perovskite films result in efficient and stable light-emitting devices. Nat. Commun. 2019, 10, 665.
Cao, F. R.; Li, L. Progress of lead-free halide perovskites: From material synthesis to photodetector application. Adv. Funct. Mater. 2021, 31, 2008275.
Xu, L. M.; Chen, J. W.; Song, J. Z.; Li, J. H.; Xue, J.; Dong, Y. H.; Cai, B.; Shan, Q. S.; Han, B. N.; Zeng, H. B. Double-protected all-inorganic perovskite nanocrystals by crystalline matrix and silica for triple-modal anti-counterfeiting codes. ACS Appl. Mater. Interfaces 2017, 9, 26556–26564.
Zhou, Y. Y.; Zhao, Y. X. Chemical stability and instability of inorganic halide perovskites. Energy Environ. Sci. 2019, 12, 1495–1511.
Fakharuddin, A.; Shabbir, U.; Qiu, W. M.; Iqbal, T.; Sultan, M.; Heremans, P.; Schmidt-Mende, L. Inorganic and layered perovskites for optoelectronic devices. Adv. Mater. 2019, 31, 1807095.
Feng, P. F.; Yang, X. X.; Feng, X. X.; Zhao, G. D.; Li, X. C.; Cao, J.; Tang, Y.; Yan, C. H. Highly stable perovskite quantum dots modified by europium complex for dual-responsive optical encoding. ACS Nano 2021, 15, 6266–6275.
Hassan, Y.; Park, J. H.; Crawford, M. L.; Sadhanala, A.; Lee, J.; Sadighian, J. C.; Mosconi, E.; Shivanna, R.; Radicchi, E.; Jeong, M. et al. Ligand-engineered bandgap stability in mixed-halide perovskite LEDs. Nature 2021, 591, 72–77.
Zhang, Q. G.; Wang, B.; Zheng, W. L.; Kong, L.; Wan, Q.; Zhang, C. Y.; Li, Z. C.; Cao, X. Y.; Liu, M. M.; Li, L. Ceramic-like stable CsPbBr3 nanocrystals encapsulated in silica derived from molecular sieve templates. Nat. Commun. 2020, 11, 31.
Hou, J. W.; Wang, Z. L.; Chen, P.; Chen, V.; Cheetham, A. K.; Wang, L. Z. Intermarriage of halide perovskites and metal-organic framework crystals. Angew. Chem., Int. Ed. 2020, 59, 19434–19449.
Song, W. T.; Wang, D. D.; Tian, J. W.; Qi, G. B.; Wu, M.; Liu, S. T.; Wang, T. T.; Wang, B.; Yao, Y. F.; Zou, Z. G. et al. Encapsulation of dual-passivated perovskite quantum dots for bio-imaging. Small 2022, 18, 2204763.
Lv, W. Z.; Li, L.; Xu, M. C.; Hong, J. X.; Tang, X. X.; Xu, L. G.; Wu, Y. H.; Zhu, R.; Chen, R. F.; Huang, W. Improving the stability of metal halide perovskite quantum dots by encapsulation. Adv. Mater. 2019, 31, 1900682.
Dirin, D. N.; Benin, B. M.; Yakunin, S.; Krumeich, F.; Raino, G.; Frison, R.; Kovalenko, M. V. Microcarrier-assisted inorganic shelling of lead halide perovskite nanocrystals. ACS Nano 2019, 13, 11642–11652.
Shamsi, J.; Urban, A. S.; Imran, M.; De Trizio, L.; Manna, L. Metal halide perovskite nanocrystals: Synthesis, post-synthesis modifications, and their optical properties. Chem. Rev. 2019, 119, 3296–3348.
Ruan, L. F.; Zhang, Y. NIR-excitable heterostructured upconversion perovskite nanodots with improved stability. Nat. Commun. 2021, 12, 219.
Zhang, Q.; Song, Y. H.; Hao, J. M.; Lan, Y. F.; Feng, L. Z.; Ru, X. C.; Wang, J. J.; Song, K. H.; Yang, J. N.; Chen, T. et al. α-BaF2 nanoparticle substrate-enabled γ-CsPbI3 heteroepitaxial growth for efficient and bright deep-red light-emitting diodes. J. Am. Chem. Soc. 2022, 144, 8162–8170.
Imran, M.; Peng, L. C.; Pianetti, A.; Pinchetti, V.; Ramade, J.; Zito, J.; Di Stasio, F.; Buha, J.; Toso, S.; Song, J. et al. Halide perovskite-lead chalcohalide nanocrystal heterostructures. J. Am. Chem. Soc. 2021, 143, 1435–1446.
Wei, Y.; Li, K.; Cheng, Z. Y.; Liu, M. M.; Xiao, H.; Dang, P. P.; Liang, S. S.; Wu, Z. J.; Lian, H. Z.; Lin, J. Epitaxial growth of CsPbX3 (X = Cl, Br, I) perovskite quantum dots via surface chemical conversion of Cs2GeF6 double perovskites: A novel strategy for the formation of leadless hybrid perovskite phosphors with enhanced stability. Adv. Mater. 2019, 31, 1807592.
Khan, W. U.; Zhou, P.; Qin, L. Y.; Alam, A.; Ge, Z. J.; Wang, Y. H. Solvent-free synthesis of nitrogen doped carbon dots with dual emission and their biological and sensing applications. Mater. Today Nano 2022, 18, 100205.
Liu, J. Q.; Pei, L.; Xia, Z. G.; Xu, Y. Hierarchical accordion-like lanthanide-based metal-organic frameworks: Solvent-free syntheses and ratiometric luminescence temperature-sensing properties. Cryst. Growth Des. 2019, 19, 6586–6591.
Xu, L. F.; Liu, J. Q.; Pei, L.; Xu, Y.; Xia, Z. G. Enhanced up-conversion luminescence and optical temperature sensing in graphitic C3N4 quantum dots grafted with BaWO4: Yb3+, Er3+ phosphors. J. Mater. Chem. C 2019, 7, 6112–6119.
Jana, A.; Mittal, M.; Singla, A.; Sapra, S. Solvent-free, mechanochemical syntheses of bulk trihalide perovskites and their nanoparticles. Chem. Commun. 2017, 53, 3046–3049.
Lou, S. Q.; Zhou, Z.; Xuan, T. T.; Li, H. L.; Jiao, J.; Zhang, H. W.; Gautier, R.; Wang, J. Chemical transformation of lead halide perovskite into insoluble, less cytotoxic, and brightly luminescent CsPbBr3/CsPb2Br5 composite nanocrystals for cell imaging. ACS Appl. Mater. Interfaces 2019, 11, 24241–24246.
Wang, L.; Ma, D. C.; Guo, C.; Jiang, X.; Li, M. L.; Xu, T. T.; Zhu, J. P.; Fan, B. B.; Liu, W.; Shao, G. et al. CsPbBr3 nanocrystals prepared by high energy ball milling in one-step and structural transformation from CsPbBr3 to CsPb2Br5. Appl. Surf. Sci. 2021, 543, 148782.
Zhong, Q. X.; Liu, J.; Chen, S. H.; Li, P. L.; Chen, J. N.; Guan, W. H.; Qiu, Y. H.; Xu, Y.; Cao, M. H.; Zhang, Q. Highly stable CsPbX3/PbSO4 core/shell nanocrystals synthesized by a simple post-treatment strategy. Adv. Opt. Mater. 2021, 9, 2001763.
Li, W.; Zhan, J.; Liu, X. R.; Tang, J. F.; Yin, W. J.; Prezhdo, O. V. Atomistic mechanism of passivation of halide vacancies in lead halide perovskites by alkali ions. Chem. Mater. 2021, 33, 1285–1292.
Huang, Z. P.; Ma, B.; Wang, H.; Li, N.; Liu, R. T.; Zhang, Z. Q.; Zhang, X. D.; Zhao, J. H.; Zheng, P. Z.; Wang, Q. et al. In situ growth of 3D/2D (CsPbBr3/CsPb2Br5) perovskite heterojunctions toward optoelectronic devices. J. Phys. Chem. Lett. 2020, 11, 6007–6015.
Tang, X. S.; Yang, J.; Li, S. Q.; Liu, Z. Z.; Hu, Z. P.; Hao, J. Y.; Du, J.; Leng, Y. X.; Qin, H. Y.; Lin, X. et al. Single halide perovskite/semiconductor core/shell quantum dots with ultrastability and nonblinking properties. Adv. Sci. 2019, 6, 1900412.
Jiang, G. C.; Guhrenz, C.; Kirch, A.; Sonntag, L.; Bauer, C.; Fan, X. L.; Wang, J.; Reineke, S.; Gaponik, N.; Eychmuller, A. Highly luminescent and water-resistant CsPbBr3-CsPb2Br5 perovskite nanocrystals coordinated with partially hydrolyzed poly(methyl methacrylate) and polyethylenimine. ACS Nano 2019, 13, 10386–10396.
Fukuda, Y.; Sanada, N.; Suzuki, Y.; Goto, T.; Nagoshi, M.; Syono, Y.; Tachiki, M. Core-level electronic states of the YBa2Cu3OyBrx superconductor studied by X-ray photoelectron spectroscopy. Phys. Rev. B 1993, 47, 418–421.
Yue, D.; Chen, D.; Lu, W.; Wang, M. N.; Zhang, X. L.; Wang, Z. L.; Qian, G. D. Enhanced photocatalytic performance and morphology evolvement of PbWO4 dendritic nanostructures through Eu3+ doping. RSC Adv. 2016, 6, 81447–81453.
Taru Chanu, T. T.; Singh, N. R. Influence of Sm3+ concentration on structural and spectroscopic properties of orange-red emitting PbWO4 phosphor: An energy transfer study. J. Solid State Chem. 2020, 284, 121190.
Kim, J. Y.; Shim, K. I.; Han, J. W.; Joo, J.; Heo, N. H.; Seff, K. Quantum dots of [Na4Cs6PbBr4]8+, water stable in zeolite X, luminesce sharply in the green. Adv. Mater. 2020, 32, 2001868.
Liu, J.; Zhong, Q. X.; Chen, S. H.; Guan, W. H.; Qiu, Y. H.; Yang, D.; Cao, M. H.; Zhang, Q. One-pot reprecipitation strategy to synthesize CsPbX3/Pb3(PO4)2 composite nanocrystals. J. Mater. Chem. C 2021, 9, 466–471.
Wang, Y. D.; Yang, X. Y.; Yu, X. P.; Duan, J. L.; Yang, Q. M.; Duan, Y. Y.; Tang, Q. W. Triboelectric charging behaviors and photoinduced enhancement of alkaline earth ions doped inorganic perovskite triboelectric nanogenerators. Nano Energy 2020, 77, 105280.
Zhang, X. L.; Xu, B.; Zhang, J. B.; Gao, Y.; Zheng, Y. J.; Wang, K.; Sun, X. W. All-inorganic perovskite nanocrystals for high-efficiency light emitting diodes: Dual-phase CsPbBr3-CsPb2Br5 composites. Adv. Funct. Mater. 2016, 26, 4595–4600.
Osherov, A.; Feldman, Y.; Kaplan-Ashiri, I.; Cahen, D.; Hodes, G. Halide diffusion in MAPbX3: Limits to topotaxy for halide exchange in perovskites. Chem. Mater. 2020, 32, 4223–4231.
Ruan, L. F.; Lin, J.; Shen, W.; Deng, Z. T. Ligand-mediated synthesis of compositionally related cesium lead halide CsPb2X5 nanowires with improved stability. Nanoscale 2018, 10, 7658–7665.
Bao, S.; Yu, H. Y.; Gao, G. Y.; Zhu, H. Y.; Wang, D. S.; Zhu, P. F.; Wang, G. F. Rare-earth single atom based luminescent composite nanomaterials: Tunable full-color single phosphor and applications in WLEDs. Nano Res. 2022, 15, 3594–3605.
Zhang, F. Y.; Hu, D. H.; Su, X. L.; Hong, Z. D.; Feng, W.; Xu, M.; Li, F. Y. Two birds with one stone: Amine-functionalized MSNs@Eu(OH)CO3 nanoprobe for efficient dissolution-enhanced afterglow bioassay. Nano Res. 2022, 15, 8360–8366.
Song, P. J.; Qiao, B.; Song, D. D.; Cao, J. Y.; Shen, Z. H.; Xu, Z.; Zhao, S. L.; Wageh, S.; Al-Ghamdi, A. Modifying the crystal field of CsPbCl3: Mn2+ nanocrystals by co-doping to enhance its red emission by a hundredfold. ACS Appl. Mater. Interfaces 2020, 12, 30711–30719.
Li, K.; Liu, X. M.; Zhang, Y.; Li, X. J.; Lian, H. Z.; Lin, J. Host-sensitized luminescence properties in CaNb2O6:Ln3+ (Ln3+ = Eu3+/Tb3+/Dy3+/Sm3+) phosphors with abundant colors. Inorg. Chem. 2015, 54, 323–333.
Dewangan, P.; Bisen, D. P.; Brahme, N.; Sharma, S.; Tamrakar, R. K.; Sahu, I. P.; Upadhyay, K. Influence of Dy3+ concentration on spectroscopic behaviour of Sr3MgSi2O8:Dy3+ phosphors. J. Alloys Compd. 2020, 816, 152590.
Ouertani, G.; Ferhi, M.; Horchani-Naifer, K.; Ferid, M. Effect of Sm3+ concentration and excitation wavelength on spectroscopic properties of GdPO4:Sm3+ phosphor. J. Alloys Compd. 2021, 885, 161178.
Pan, G. C.; Bai, X.; Yang, D. W.; Chen, X.; Jing, P. T.; Qu, S. N.; Zhang, L. J.; Zhou, D. L.; Zhu, J. Y.; Xu, W. et al. Doping lanthanide into perovskite nanocrystals: Highly improved and expanded optical properties. Nano Lett. 2017, 17, 8005–8011.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 22171040, 51932009 and 52172166), the Fundamental Research Funds for the Central Universities, China (No. N2105006). The authors are grateful to Maxim S. Molokeev from Federal Research Center KSC SB RAS for his help on discussion of lattice-matched epitaxial growth mechanism.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2023_5922_MOESM1_ESM.pdf
Structural engineering of BaWO4/CsPbX3/CsPb2X5 (X = Cl, Br, I) heterostructures towards ultrastable and tunable photoluminescence
Rights and permissions
About this article
Cite this article
Liu, P., Xu, Y., Li, B. et al. Structural engineering of BaWO4/CsPbX3/CsPb2X5 (X = Cl, Br, I) heterostructures towards ultrastable and tunable photoluminescence. Nano Res. 17, 1636–1645 (2024). https://doi.org/10.1007/s12274-023-5922-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5922-5