Abstract
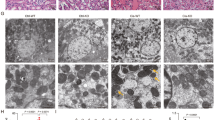
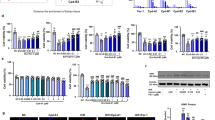
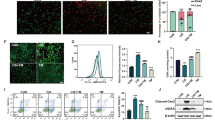
Acute kidney injury (AKI) is a heterogeneous clinical complication with no existing definite or particular therapies. Therefore, molecular mechanisms and approaches for treating acute kidney injury are in urgent need. Herein, we demonstrated that dexrazoxane (DXZ), a U.S. Food and Drug Administration (FDA)-approved cardioprotective drug, can both functionally and histologically attenuate cisplatin or ischemia-reperfusion injury-induced AKI in vitro and in vivo via inhibiting ferroptosis specifically. This effect is characterized by decreasing lipid peroxidation, shown by the biomarker of oxidative stress 4-hydroxynonenal (HNE) and prostaglandinendoperoxide synthase 2 (Ptgs2), while reversing the downregulation of glutathione peroxidase 4 (GPX4) and ferritin 1 (FTH-1). Mechanistically, the results revealed that DXZ targeted at the renal tubule significantly inhibits ferroptosis by suppressing α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD). Furthermore, the conjugation of dexrazoxane and polysialic acid (DXZ-PSA) is specifically designed and utilized to enhance the therapeutic effect of DXZ by long-term effect in the kidney, especially retention and targeting in the renal tubules. This study provides a novel therapeutic approach and mechanistic insight for AKI by inhibiting ferroptosis through a new type drug DXZ-PSA with the enhanced renal distribution.

Similar content being viewed by others
References
Bellomo, R.; Kellum, J. A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766.
Kellum, J. A.; Prowle, J. R. Paradigms of acute kidney injury in the intensive care setting. Nat. Rev. Nephrol. 2018, 14, 217–230.
Kaushal, G. P.; Shah, S. V. Challenges and advances in the treatment of AKI. J. Am. Soc. Nephrol. 2014, 25, 877–883.
Xu, Y. F.; Ma, H. B.; Shao, J.; Wu, J. F.; Zhou, L. Y.; Zhang, Z. R.; Wang, Y. Z.; Huang, Z.; Ren, J. M.; Liu, S. H. et al. A role for tubular necroptosis in cisplatin-induced AKI. J. Am. Soc. Nephrol. 2015, 26, 2647–2658.
Linkermann, A.; Skouta, R.; Himmerkus, N.; Mulay, S. R.; Dewitz, C.; De Zen, F.; Prokai, A.; Zuchtriegel, G.; Krombach, F.; Welz, P. S. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16836–16841.
Stockwell, B. R.; Angeli, J. P. F.; Bayir, H.; Bush, A. I.; Conrad, M.; Dixon, S. J.; Fulda, S.; Gascón, S.; Hatzios, S. K.; Kagan, V. E. et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285.
Hu, Z. X.; Zhang, H.; Yi, B.; Yang, S. K.; Liu, J.; Hu, J.; Wang, J. W.; Cao, K.; Zhang, W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020, 11, 73.
Wang, Y.; Quan, F.; Cao, Q. H.; Lin, Y. T.; Yue, C. X.; Bi, R.; Cui, X. M.; Yang, H. B.; Yang, Y.; Birnbaumer, L. et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2020, 28, 231–243.
Chen, C. A.; Wang, D. K.; Yu, Y. Y.; Zhao, T. Y.; Min, N. N.; Wu, Y.; Kang, L. C.; Zhao, Y.; Du, L. F.; Zhang, M. Z. et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021, 12, 65.
Sharma, S.; Leaf, D. E. Iron chelation as a potential therapeutic strategy for AKI prevention. J. Am. Soc. Nephrol. 2019, 30, 2060–2071.
Martines, A. M. F.; Masereeuw, R.; Tjalsma, H.; Hoenderop, J. G.; Wetzels, J. F. M.; Swinkels, D. W. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat. Rev. Nephrol. 2013, 9, 385–398.
Fang, X. X.; Wang, H.; Han, D.; Xie, E. J.; Yang, X.; Wei, J. Y.; Gu, S. S.; Gao, F.; Zhu, N. L.; Yin, X. J. et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2011, 116, 2672–2680.
Eneh, C.; Lekkala, M. R. Dexrazoxane; StatPearls Publishing: Treasure Island, 2022.
Karlstetter, M.; Kopatz, J.; Aslanidis, A.; Shahraz, A.; Caramoy, A.; Linnartz-Gerlach, B.; Lin, Y. C.; Lückoff, A.; Fauser, S.; Düker, K. et al. Polysialic acid blocks mononuclear phagocyte reactivity, inhibits complement activation, and protects from vascular damage in the retina. EMBO Mol. Med. 2017, 9, 154–166.
Li, J. H.; Tang, Y.; Tang, P. M. K.; Lv, J.; Huang, X. R.; Carlsson-Skwirut, C.; Da Costa, L.; Aspesi, A.; Fröhlich, S.; Szczęśniak, P. et al. Blocking macrophage migration inhibitory factor protects against cisplatin-induced acute kidney injury in mice. Mol. Ther. 2018, 26, 2523–2532.
Skrypnyk, N. I.; Harris, R. C.; De Caestecker, M. D. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J. Vis. Eop. 2013, 50495.
Li, W.; Wang, C. S.; Lv, H.; Wang, Z. H.; Zhao, M.; Liu, S. Y.; Gou, L. P.; Zhou, Y.; Li, J.; Zhang, J. Y. et al. A DNA nanoraft-based cytokine delivery platform for alleviation of acute kidney injury. ACS Nano 2021, 15, 18237–18249.
Xie, X. S.; Zhang, Y. J.; Su, X. W.; Wang, J. N.; Yao, X.; Lv, D.; Zhou, Q.; Mao, J. H.; Chen, J. H.; Han, F. et al. Targeting iron metabolism using gallium nanoparticles to suppress ferroptosis and effectively mitigate acute kidney injury. Nano Res. 2022, 15, 6315–6327.
Prus, E.; Fibach, E. Flow cytometry measurement of the labile iron pool in human hematopoietic cells. Cytometry A 2008, 73, 22–27.
Gao, M. H.; Yi, J. M.; Zhu, J. J.; Minikes, A. M.; Monian, P.; Thompson, C. B.; Jiang, X. J. Role of Mitochondria in ferroptosis. Mol. Cell 2011, 73, 354–363.e3.
Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X. X.; Freitas, F. P.; Seibt, T. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018, 172, 409–422.e21.
Gao, M. H.; Monian, P.; Pan, Q. H.; Zhang, W.; Xiang, J.; Jiang, X. J. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032.
Tian, Y.; Lu, J.; Hao, X. Q.; Li, H.; Zhang, G. Y.; Liu, X. L.; Li, X. R.; Zhao, C. P.; Kuang, W. H.; Chen, D. F. et al. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease. Neurotherapeutics 2020, 17, 1796–1812.
Van Biesen, W.; Vanholder, R.; Lameire, N. Defining acute renal failure: RIFLE and beyond. Clin. J. Am. Soc. Nephrol. 2006, 1, 1314–1319.
Bellomo, R.; Kellum, J. A.; Ronco, C. Defining and classifying acute renal failure: From advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007, 33, 409–413.
Bauckman, K. A.; Mysorekar, I. U. Ferritinophagy drives uropathogenic Escherichia coli persistence in bladder epithelial cells. Autophagy 2016, 12, 850–863.
Yoshino, J. ACMSD: A novel target for modulating NAD+ homeostasis. Trends Endocrinol. Metab. 2019, 30, 229–232.
Wang, H. Y.; Cheng, Y.; Mao, C.; Liu, S.; Xiao, D. S.; Huang, J.; Tao, Y. G. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 2021, 29, 2185–2208.
Yang, W. S.; Stockwell, B. R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol 2016, 26, 165–176.
Cao, H. M.; Cheng, Y. Q.; Gao, H. Q.; Zhuang, J.; Zhang, W. G.; Bian, Q.; Wang, F.; Du, Y.; Li, Z. J.; Kong, D. L. et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano 2020, 14, 4014–4026.
Maiorino, M.; Conrad, M.; Ursini, F. GPx4, lipid peroxidation, and cell death: Discoveries, rediscoveries, and open issues. Antioxid. Redox Signal. 2018, 29, 61–74.
Mühlenhoff, M.; Eckhardt, M.; Gerardy-Schahn, R. Polysialic acid: Three-dimensional structure, biosynthesis and function. Curr. Opin. Struct. Biol. 1998, 8, 558–564.
Troy, F. A. Polysialylation: From bacteria to brains. Glycobiology 1992, 2, 5–23.
Katsyuba, E.; Mottis, A.; Zietak, M.; De Franco, F.; Van Der Velpen, V.; Gariani, K.; Ryu, D.; Cialabrini, L.; Matilainen, O.; Liscio, P. et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 2018, 563, 354–359.
Li, T. F.; Walker, A. L.; Iwaki, H.; Hasegawa, Y.; Liu, A. M. Kinetic and spectroscopic characterization of ACMSD from Pseudomonas fluorescens reveals a pentacoordinate mononuclear metallocofactor. J. Am. Chem. Soc. 2005, 127, 12282–12290.
Ooi, A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Semin. Cancer Biol. 2020, 61, 158–166.
Shan, L.; Xu, X. M.; Zhang, J.; Cai, P.; Gao, H.; Lu, Y. J.; Shi, J. G.; Guo, Y. L.; Su, Y. Increased hemoglobin and heme in MALDI-TOF MS analysis induce ferroptosis and promote degeneration of herniated human nucleus pulposus. Mol. Med. 2021, 27, 103.
Liu, P. F.; Wu, D.; Duan, J. Y.; Xiao, H. X.; Zhou, Y. L.; Zhao, L.; Feng, Y. T. NRF2 regulates the sensitivity of human NSCLC cells to cystine deprivation-induced ferroptosis via FOCAD-FAK signaling pathway. Redox Biol. 2020, 37, 101702.
Acknowledgments
This study was supported by the Core Facility, Zhejiang University School of Medicine. This work was supported by grants from Zhejiang Provincial Natural Science Foundation of China (No. LZ22H050001), the National Natural Science Foundation of China (Nos. 81970573, 81670651, 81900683, 82000637, and 82173662), Zhejiang provincial program for the Cultivation of High-level Innovative Health talents, Natural Science Foundation of Shanghai (No. 20ZR1410400) and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2020KY538).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, Y., Wu, J., An, Q. et al. Renal tubule-targeted dexrazoxane suppresses ferroptosis in acute kidney injury by inhibiting ACMSD. Nano Res. 16, 9701–9714 (2023). https://doi.org/10.1007/s12274-023-5547-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5547-8