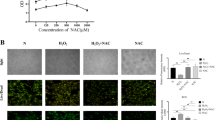
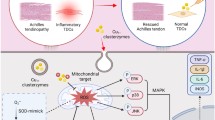
Abstract
Background
Reactive oxygen species (ROS) is considered as ubiquitous and highly active chemicals that influence tendon integrity and orchestrate tendon repair. With significant recent advances in nanomaterials, cerium oxide nanoparticles (CeO2 NPs) exhibit superoxide dismutase- and catalase-like activities. Herein, we introduced a therapeutic approach of CeO2 NPs for Achilles tendinopathy (AT) healing.
Methods
CeO2 NPs were synthesized to examine their effect as ROS scavengers on AT healing in vitro and in vivo. The mRNA levels of inflammatory factors were evaluated in AT after CeO2 NPs treatment in vitro. The mechanisms underlying CeO2 NPs-mediated stimulation of NRF2 translocation and ERK signaling were verified through immunofluorescence and Western blot analysis. The efficacy of CeO2 NPs was tested in an AT rat model in comparison with the control.
Results
CeO2 NPs not only significantly scavenged multiple ROS and suppressed ROS-induced inflammatory reactions but also protected cell proliferation under oxidative stress induced by tert-butyl hydroperoxide (TBHP). Moreover, CeO2 NPs could promote NRF2 nuclear translocation for anti-oxidation and anti-inflammation through the ERK signaling pathway. In a rat model of collagenase-induced tendon injuries, CeO2 NPs showed significant therapeutic efficacy by ameliorating tendon damage.
Conclusion
The present study provides valuable insights into the molecular mechanism of CeO2 NPs to ameliorate ROS in tenocytes via the ERK/NRF2 signaling pathway, which underscores the potential of CeO2 NPs for application in the treatment of enthesopathy healing.

Similar content being viewed by others
References
De Jonge, S.; Van Den Berg, C.; De Vos, R. J.; Van Der Heide, H. J. L.; Weir, A.; Verhaar, J. A. N.; Bierma-Zeinstra, S. M. A.; Tol, J. L. Incidence of midportion Achilles tendinopathy in the general population. Br. J. Sports Med. 2011, 45, 1026–1028.
Magnusson, S. P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268.
Vo, T. P.; Ho, G. W. K.; Andrea, J. Achilles tendinopathy, a brief review and update of current literature. Curr. Sports Med. Rep. 2021, 20, 453–461.
Murrell, G. A. C. Oxygen free radicals and tendon healing. J. Shoulder Elbow Surg. 2007, 16, S208–S214.
Millar, N. L.; Murrell, G. A. C.; McInnes, I. B. Alarmins in tendinopathy: Unravelling new mechanisms in a common disease. Rheumatology (Oxford). 2013, 52, 769–779.
Bestwick, C. S.; Maffulli, N. Reactive oxygen species and tendinopathy: Do they matter? Br. J. Sports Med. 2004, 38, 672–674.
Millar, N. L.; Murrell, G. A. C.; McInnes, I. B. Inflammatory mechanisms in tendinopathy—towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122.
Li, X. Y.; Fang, P.; Mai, J.; Choi, E. T.; Wang, H.; Yang, X. F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19.
Yao, J.; Cheng, Y.; Zhou, M.; Zhao, S.; Lin, S. C.; Wang, X. Y.; Wu, J. J. X.; Li, S. R.; Wei, H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018, 9, 2927–2933.
Korsvik, C.; Patil, S.; Seal, S.; Self, W. T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058.
Zhao, S.; Li, Y. X.; Liu, Q. Y.; Li, S. R.; Cheng, Y.; Cheng, C. Q.; Sun, Z. Y.; Du, Y.; Butch, C. J.; Wei, H. An orally administered CeO2@montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv. Funct. Mater. 2020, 30, 2004692.
Yu, Y. J.; Zhao, S.; Gu, D. A.; Zhu, B. J.; Liu, H. X.; Wu, W. L.; Wu, J. J. X.; Wei, H.; Miao, L. Y. Cerium oxide nanozyme attenuates periodontal bone destruction by inhibiting the ROS-NF κB pathway. Nanoscale 2022, 14, 2628–2637.
Lin, A. Q.; Liu, Q. Y.; Zhang, Y. H.; Wang, Q.; Li, S. R.; Zhu, B. J.; Miao, L. Y.; Du, Y.; Zhao, S.; Wei, H. A dopamine-enabled universal assay for catalase and catalase-like nanozymes. Anal. Chem. 2022, 94, 10636–10642.
Farías, G. G.; Guardia, C. M.; Britt, D. J.; Guo, X. L.; Bonifacino, J. S. Sorting of dendritic and axonal vesicles at the pre-axonal exclusion zone. Cell Rep. 2015, 13, 1221–1232.
Rizvi, F.; Shukla, S.; Kakkar, P. Essential role of pH domain and leucine-rich repeat protein phosphatase 2 in Nrf2 suppression via modulation of Akt/GSK3β/Fyn kinase axis during oxidative hepatocellular toxicity. Cell Death Dis. 2014, 5, e1153.
Millar, N. L.; Silbernagel, K. G.; Thorborg, K.; Kirwan, P. D.; Galatz, L. M.; Abrams, G. D.; Murrell, G. A. C.; McInnes, I. B.; Rodeo, S. A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1.
Challoumas, D.; Biddle, M.; Millar, N. L. Recent advances in tendinopathy. Fac. Rev. 2020, 9, 16.
Riley, G. Chronic tendon pathology: Molecular basis and therapeutic implications. Expert Rev. Mol. Med. 2005, 7, 1–25.
D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surgeon 2017, 15, 297–302.
Zhang, X. Y.; Eliasberg, C. D.; Rodeo, S. A. Mitochondrial dysfunction and potential mitochondrial protectant treatments in tendinopathy. Ann. N Y Acad. Sci. 2021, 1490, 29–41.
Liu, Y. C.; Wang, H. L.; Huang, Y. Z.; Weng, Y. H.; Chen, R. S.; Tsai, W. C.; Yeh, T. H.; Lu, C. S.; Chen, Y. L.; Lin, Y. W. et al. Alda-1, an activator of ALDH2, ameliorates Achilles tendinopathy in cellular and mouse models. Biochem. Pharmacol. 2020, 175, 113919.
Lee, J. M.; Hwang, J. W.; Kim, M. J.; Jung, S. Y.; Kim, K. S.; Ahn, E. H.; Min, K.; Choi, Y. S. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants (Basel) 2021, 10, 696.
Li, K. Q.; Deng, G. M.; Deng, Y.; Chen, S. W.; Wu, H. T.; Cheng, C. Y.; Zhang, X. R.; Yu, B.; Zhang, K. R. High cholesterol inhibits tendon-related gene expressions in tendon-derived stem cells through reactive oxygen species-activated nuclear factor-κB signaling. J. Cell. Physiol. 2019, 234, 18017–18028.
Kucukler, S.; Benzer, F.; Yildirim, S.; Gur, C.; Kandemir, F. M.; Bengu, A. S.; Ayna, A.; Caglayan, C.; Dortbudak, M. B. Protective effects of chrysin against oxidative stress and inflammation induced by lead acetate in rat kidneys: A biochemical and histopathological approach. Biol. Trace Elem. Res. 2021, 199, 1501–1514.
Yardım, A.; Kandemir, F. M.; Çomaklı, S.; Özdemir, S.; Caglayan, C.; Kucukler, S.; Çelik, H. Protective effects of curcumin against paclitaxel-induced spinal cord and sciatic nerve injuries in rats. Neurochem. Res. 2021, 46, 379–395.
Wei, H.; Wang, E. K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093.
Szymanski, C. J.; Munusamy, P.; Mihai, C.; Xie, Y. M.; Hu, D. H.; Gilles, M. K.; Tyliszczak, T.; Thevuthasan, S.; Baer, D. R.; Orr, G. Shifts in oxidation states of cerium oxide nanoparticles detected inside intact hydrated cells and organelles. Biomaterials 2015, 62, 147–154.
Kwon, H. J.; Kim, D.; Seo, K.; Kim, Y. G.; Han, S. I.; Kang, T.; Soh, M.; Hyeon, T. Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson’s disease. Angew. Chem., Int. Ed. 2018, 57, 9408–9412.
Bao, Q. Q.; Hu, P.; Xu, Y. Y.; Cheng, T. S.; Wei, C. Y.; Pan, L. M.; Shi, J. L. Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano 2018, 12, 6794–6805.
Son, D.; Lee, J.; Lee, D. J.; Ghaffari, R.; Yun, S. M.; Kim, S. J.; Lee, J. E.; Cho, H. R.; Yoon, S.; Yang, S. et al. Bioresorbable electronic stent integrated with therapeutic nanoparticles for endovascular diseases. ACS Nano 2015, 9, 5937–5946.
Kim, J.; Hong, G.; Mazaleuskaya, L.; Hsu, J. C.; Rosario-Berrios, D. N.; Grosser, T.; Cho-Park, P. F.; Cormode, D. P. Ultrasmall antioxidant cerium oxide nanoparticles for regulation of acute inflammation. ACS Appl. Mater. Interfaces 2021, 13, 60852–60864.
Adebayo, O. A.; Akinloye, O.; Adaramoye, O. A. Cerium oxide nanoparticles attenuate oxidative stress and inflammation in the liver of diethylnitrosamine-treated mice. Biol. Trace Elem. Res. 2020, 193, 214–225.
Soh, M.; Kang, D. W.; Jeong, H. G.; Kim, D.; Kim, D. Y.; Yang, W.; Song, C.; Baik, S.; Choi, I. Y.; Ki, S. K. et al. Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem., Int. Ed. 2017, 56, 11399–11403.
Wang, P.; Geng, J.; Gao, J. H.; Zhao, H.; Li, J. H.; Shi, Y. R.; Yang, B. Y.; Xiao, C.; Linghu, Y. Y.; Sun, X. F. et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019, 10, 755.
Tohidnezhad, M.; Varoga, D.; Wruck, C. J.; Brandenburg, L. O.; Seekamp, A.; Shakibaei, M.; Sönmez, T. T.; Pufe, T.; Lippross, S. Platelet-released growth factors can accelerate tenocyte proliferation and activate the anti-oxidant response element. Histochem. Cell Biol. 2011, 135, 453–460.
Gañán-Gómez, I.; Wei, Y.; Yang, H.; Boyano-Adánez, M. C.; García-Manero, G. Oncogenic functions of the transcription factor Nrf2. Free Radic. Biol. Med. 2013, 65, 750–764.
Carvajal, S.; Perramón, M.; Casals, G.; Oró, D.; Ribera, J.; Morales-Ruiz, M.; Casals, E.; Casado, P.; Melgar-Lesmes, P.; Fernández-Varo, G. et al. Cerium oxide nanoparticles protect against oxidant injury and interfere with oxidative mediated kinase signaling in human-derived hepatocytes. Int. J. Mol. Sci. 2019, 20, 5959.
Saita, M.; Kaneko, J.; Sato, T.; Takahashi, S. S.; Wada-Takahashi, S.; Kawamata, R.; Sakurai, T.; Lee, M. C. I.; Hamada, N.; Kimoto, K. et al. Novel antioxidative nanotherapeutics in a rat periodontitis model: Reactive oxygen species scavenging by redox injectable gel suppresses alveolar bone resorption. Biomaterials 2016, 76, 292–301.
Liu, A. L.; Wang, Q.; Zhao, Z. N.; Wu, R.; Wang, M. C.; Li, J. W.; Sun, K. Y.; Sun, Z. Y.; Lv, Z. Y.; Xu, J. et al. Nitric oxide nanomotor driving exosomes-loaded microneedles for achilles tendinopathy healing. ACS Nano 2021, 15, 13339–13350.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81941009, 81991514, 32271409, and 82202778), Nanjing Distinguished Youth Fund (No. JQX20001), Jiangsu Provincial Key R&D Program (No. BE2022836), China Postdoctoral Science Foundation (No. 2020M671456), National Basic Research Program of China (No. 2021YFA1201404), Jiangsu Provincial Key Medical Center Foundation, Jiangsu Provincial Medical Outstanding Talent Foundation, Jiangsu Provincial Medical Youth Talent Foundation and Jiangsu Provincial Key Medical Talent Foundation, and the Fundamental Research Funds for the Central Universities (Nos. 14380493 and 14380494).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xu, X., Wang, R., Li, Y. et al. Cerium oxide nanozymes alleviate oxidative stress in tenocytes for Achilles tendinopathy healing. Nano Res. 16, 7364–7372 (2023). https://doi.org/10.1007/s12274-023-5416-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5416-5